Novel photosensitizer as well as preparation method and application thereof
A photosensitizer, a new type of technology, applied in the field of photosensitizers, can solve problems such as high oxygen dependence, and achieve the effect of outstanding universality and high-efficiency treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] (1) Synthesis of compound Ⅰ
[0047]Thionyl chloride (0.3 mL, 4.0 mmol) was added to a suspension of D-biotin (300 mg, 1.23 mmol) in 10 mL of methanol, and stirred overnight at room temperature until the reaction liquid became clear and transparent. A white solid was obtained after distillation under reduced pressure. Disperse it in 10mL of methanol, slowly add hydrazine hydrate (0.48mL, 10mmol) dropwise while stirring, heat to 70°C, and react for 12h. Cool to room temperature, distill under reduced pressure, pour into a large amount of ice-cold methanol, filter with suction, and wash with methanol three times to obtain compound Ⅰ in the form of white powder.
[0048] (2) Synthesis of Compound Ⅲa
[0049] Under a nitrogen atmosphere, dissolve compound IIa (DCF-TFM, 20.4 mg, 0.025 mmol) in 5 mL of anhydrous DMF, and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene at 0°C Amine hydrochloride (EDCI) (24mg, 0.125mmol), 1-hydroxybenzotriazole (HOBt) (17mg,...
Embodiment 2
[0051]
[0052] (1) The synthesis of compound I is the same as in Example 1.
[0053] (2) Synthesis of compound Ⅲb
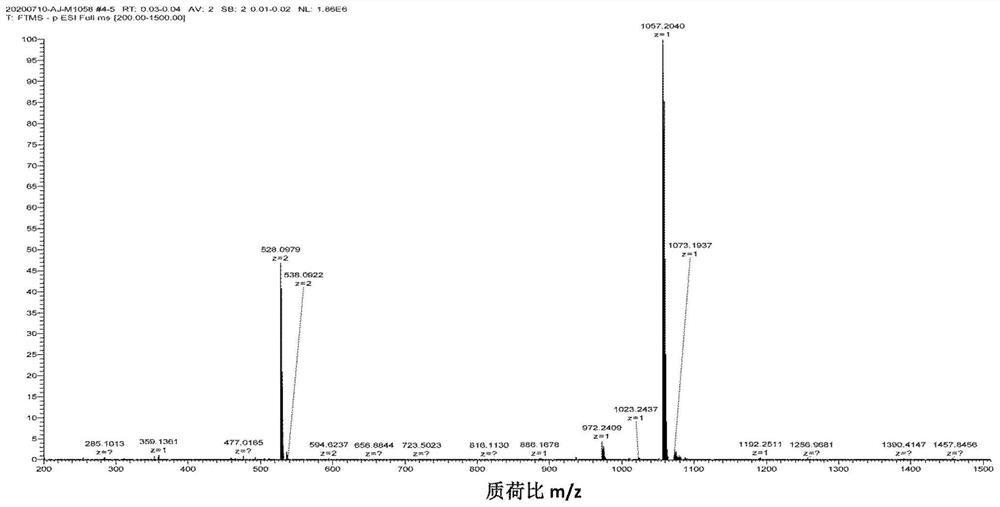
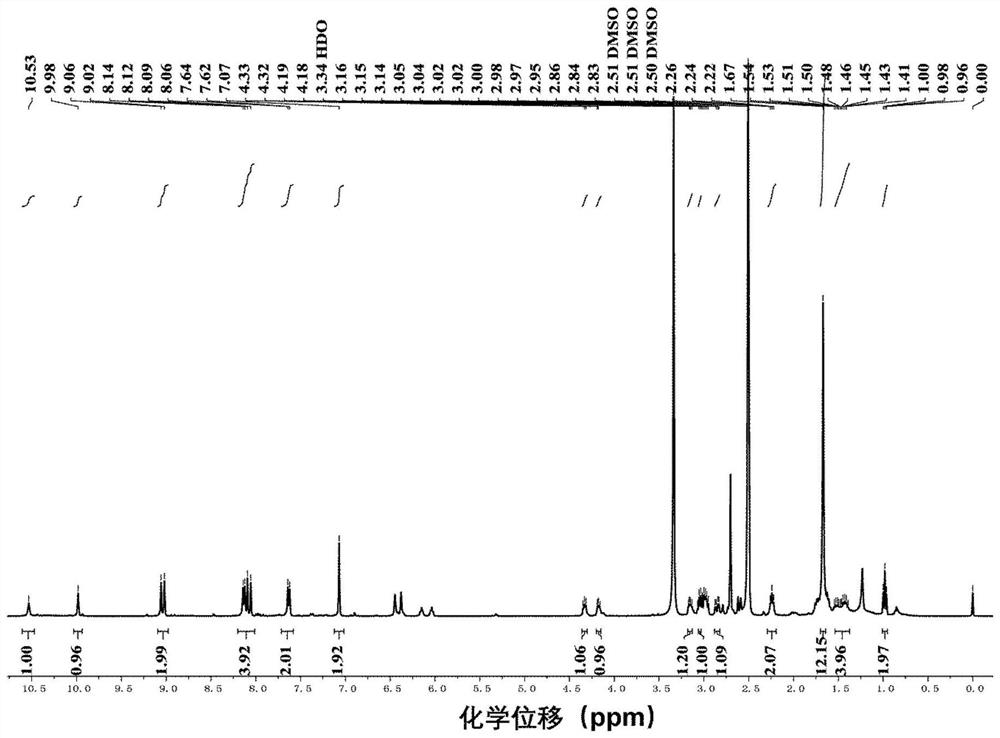
[0054] Under nitrogen atmosphere, dissolve compound Ⅱb (FL, 50mg, 0.065mmol) in 5mL of anhydrous DMF, at 0°C, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Salt (EDCI) (62.8mg, 0.33mmol), 1-hydroxybenzotriazole (HOBt) (44mg, 0.33mmol) and N,N-diisopropylethylamine (DIEA) (32μL, 0.2mmol) were reacted for 2h After that, compound Ⅰ (33.5mg, 0.13mmol) was added and stirred at room temperature for 24h. Distilled under reduced pressure and purified by column chromatography (methanol / dichloromethane system=1 / 8) to obtain photosensitizer compound IIIb. The high-resolution mass spectrometry and proton NMR spectrum characterization charts of compound Ⅲb are as follows: image 3 and Figure 4 shown.
Embodiment 3
[0056]
[0057] (1) The synthesis of compound I is the same as in Example 1.
[0058] (2) Synthesis of Compound IIIc
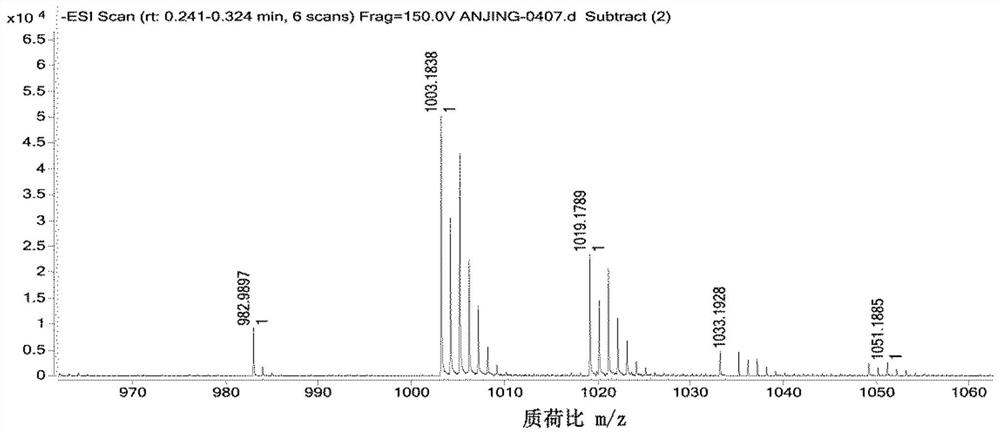
[0059] Under a nitrogen atmosphere, dissolve compound IIc (PpIX, 30 mg, 0.053 mmol) in 3 mL of anhydrous DMF, and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride at 0°C salt (EDCI) (10.56mg, 0.053mmol), 1-hydroxybenzotriazole (HOBt) (7.43mg, 0.053mmol) and N,N-diisopropylethylamine (DIEA) (8.75μL, 0.053mmol) After reacting for 2h, compound I (15.3mg, 0.05mmol) was added and stirred at room temperature for 24h. Distilled under reduced pressure and purified by column chromatography (methanol / dichloromethane system=1 / 8) to obtain photosensitizer compound IIIc. The high-resolution mass spectrometry and proton NMR spectrum characterization charts of compound Ⅲc are as follows: Figure 5 and Figure 6 shown.
[0060] Figure 7 is the absorption and emission spectra of photosensitizer compound IIIa and compound IIa in ethanol, Figure 8 is t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com