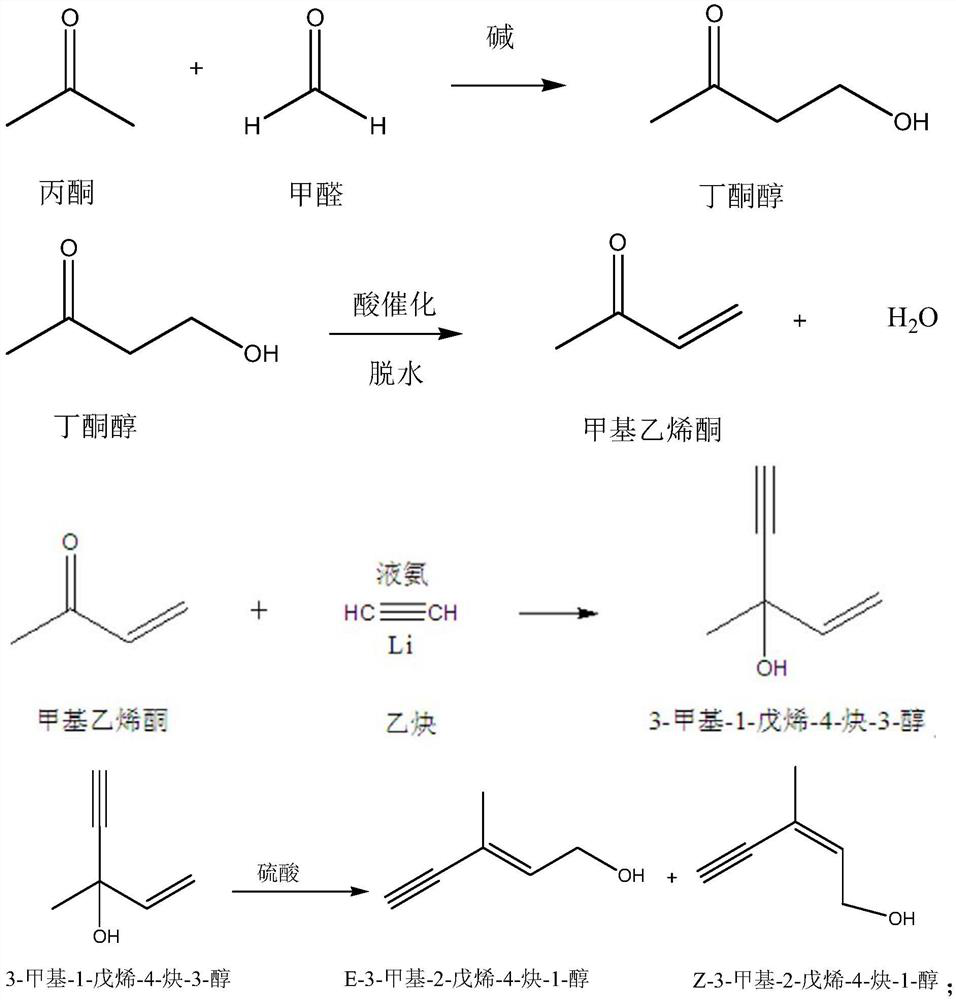
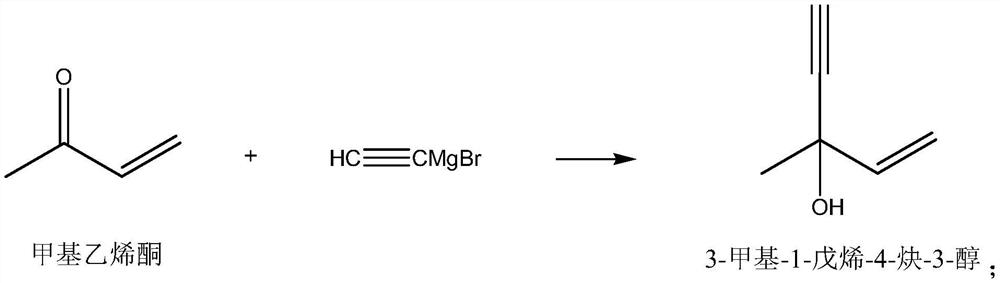
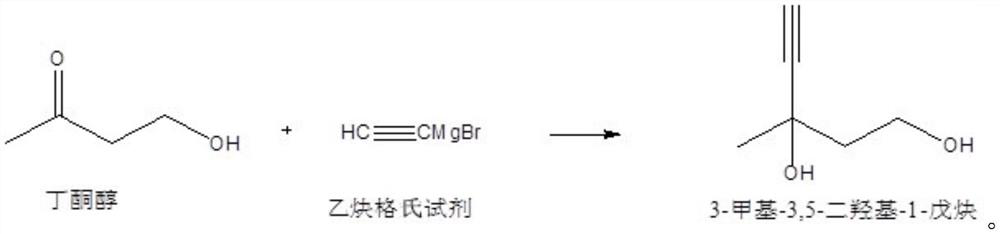
Synthesis method of vitamin A intermediate
A synthesis method and technology for intermediates, which are applied in the field of synthesis of vitamin A intermediates, can solve problems such as unsatisfactory results, and achieve the effects of reduced industrialization difficulty, easy large-scale production, and improved yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) butanone alcohol is dissolved in an organic solvent to make a butanone alcohol solution; the organic solvent is toluene, and the consumption adds 1ml by every gram of butanone alcohol;
[0059] (2) Control the temperature of the butanone alcohol solution to be 15° C. Under a nitrogen atmosphere and stirring conditions, add dropwise acetylene magnesium bromide-tetrahydrofuran solution to the butanone alcohol solution; after the addition is completed, continue to stir the reaction until the end of the reaction, and obtain Reaction solution; the concentration of acetylene magnesium bromide-tetrahydrofuran solution is 0.2M; the consumption of acetylene magnesium bromide-tetrahydrofuran solution is 1:2.2 according to the mol ratio of butanone alcohol and acetylene magnesium bromide in the whole material; The magnesium bromide-tetrahydrofuran solution was added dropwise in 2 hours; the reaction material was sampled and detected, and when the mass content of butanone alcoho...
Embodiment 2
[0069] Method is with embodiment 1, and difference is:
[0070] (1) The organic solvent is tetrahydrofuran, and the consumption is added 2ml per gram of butanone alcohol;
[0071] (2) control butanone alcohol solution temperature is 20 ℃; The concentration of acetylene magnesium bromide-tetrahydrofuran solution is 0.5M; The consumption of acetylene magnesium bromide-tetrahydrofuran solution is according to the mol ratio of butanone alcohol and acetylene magnesium bromide in the whole material The ratio is 1:2.3; the rate of addition is controlled by adding the acetylene magnesium bromide-tetrahydrofuran solution within 3 hours;
[0072] (3) The mass concentration of sulfuric acid solution is 10%; The consumption of sulfuric acid solution is 1: 6 by the volume ratio of distillate raffinate and sulfuric acid solution;
[0073] (4) The amount of dichloromethane is 1:6 according to the volume ratio of the mixed solution and dichloromethane;
[0074] (5) The solvent is n-hexane; ...
Embodiment 3
[0079] Method is with embodiment 1, and difference is:
[0080] (1) The organic solvent is n-hexane, and the consumption is added 2ml per gram of butanone alcohol;
[0081] (2) control butanone alcohol solution temperature is 25 ℃; The concentration of acetylene magnesium bromide-tetrahydrofuran solution is 0.8M; The consumption of acetylene magnesium bromide-tetrahydrofuran solution is according to the mol ratio of butanone alcohol and acetylene magnesium bromide in the whole material The ratio is 1:2.4; the rate of addition is controlled at the completion of the addition of the acetylene magnesium bromide-tetrahydrofuran solution within 3 hours;
[0082] (3) The mass concentration of sulfuric acid solution is 12%; The consumption of sulfuric acid solution is 1: 4 by the volume ratio of distillate raffinate and sulfuric acid solution;
[0083] (4) The amount of dichloromethane is 1:8 according to the volume ratio of the mixed solution and dichloromethane;
[0084] (5) The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com