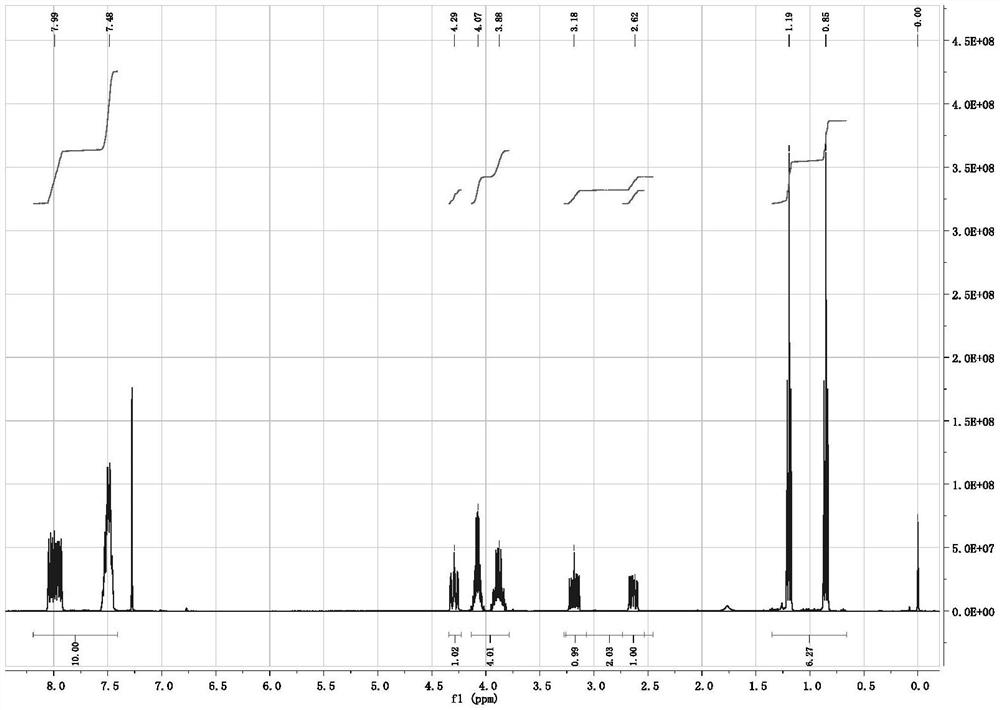
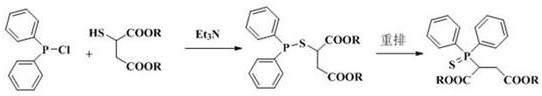
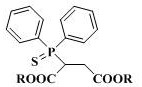
2-(diphenylthiophosphoryl) succinate as well as synthesis method and application thereof
A technology of diphenyl thiophosphoryl and diphenyl phosphono is applied in the field of industrial production of flame retardant 2-(diphenyl thiophosphoryl) succinate, which can solve the problem of high odor and unpleasantness. Suitable for large-scale production, difficult transportation and storage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]Example 1: According to the synthesis method of the document Synthetic Communications, 1980, 10(3), 175-82, 2.00 g of mercaptosuccinic acid, 20.0 mL of absolute ethanol and 3% molar equivalent of p-toluenesulfonic acid were added to a round-bottomed reaction flask Stir in medium, and react overnight under reflux in an external bath at 85°C. After most of the solvent was removed by rotary evaporation, 30 mL of ethyl acetate was added to dissolve the crude product, and then saturated sodium bicarbonate (10 mL×1) and saturated sodium chloride ( 10 mL × 2) washed until the organic phase was neutral, dried over anhydrous magnesium sulfate, filtered, and rotary evaporated to obtain 2.47 g of diethyl mercaptosuccinate with a yield of 90%, which could be directly used in the next reaction.
[0023] Under nitrogen protection, 2.00 g (9.6 mmol) of the above crude product diethyl mercaptosuccinate, 1.02 g (10.0 mmol) of triethylamine and 8.0 mL of dichloromethane were added to the r...
Embodiment 2
[0026] Add 1.78 g (10 mmol) of dimethyl mercaptosuccinate (content >98.5%), 1.05 g (10 mmol) of dry-treated triethylamine and 10 mL of dichloromethane to a 500 mL reaction under nitrogen protection In the flask, cooled in an ice bath, slowly add a mixture of 2.66 g (12.0 mmol) of diphenylphosphorus chloride and 5.0 mL of dichloromethane dropwise through a constant pressure dropping funnel, and the dropwise addition is completed within about 10 to 15 minutes, and then the temperature is raised Reaction was carried out at 60°C for 24 h, and TLC detected that the reaction of dimethyl mercaptosuccinate was complete. After the reaction was stopped, the reaction solution was quickly filtered, and the solvent was spin-dried under reduced pressure to obtain a crude product. Transfer the crude product to a sealed tube of 100 mL, dilute the product with 1.0 mL of chlorobenzene, then add 20 mg of iodine element, stir and seal under nitrogen protection, and react overnight at 140°C in an e...
Embodiment 3
[0028] Under nitrogen protection conditions, 20.0 g (97 mmol) of diethyl mercaptosuccinate (content >98.5%), 9.8 g (97 mmol) of dry-treated triethylamine and 60 mL of dichloromethane were added to a 500 mL reaction In the flask, cooled in an ice bath, slowly add a mixture of 22.4 g (101 mmol) of diphenylphosphine chloride and 20 mL of dichloromethane dropwise through a constant pressure dropping funnel, and the dropwise addition is completed within about 10 to 15 minutes, and then naturally Warming up to room temperature, reacting for 3-4 h, TLC detection of diethyl mercaptosuccinate reaction is complete, stop the reaction, quickly filter the reaction solution, and spin dry the solvent under reduced pressure to obtain the crude product. Transfer the crude product to a 100 mL sealed tube, dilute the product with 15 mL of toluene, then add 0.2 g (0.9 mmol) iodine element, stir and seal under nitrogen protection, and react overnight in an external bath at 160 ° C. After TLC detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com