Kit for evaluating curative effect of platinum drugs for patients with lung cancer or bladder cancer and application of kit
A platinum-based drug and curative effect evaluation technology, applied in the field of molecular biology, can solve the problems of huge differences in the curative effect of platinum-based drugs, affecting the treatment effect, prognosis and quality of life of patients, and achieve rigorous detection, good specificity, and reduced operational errors Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
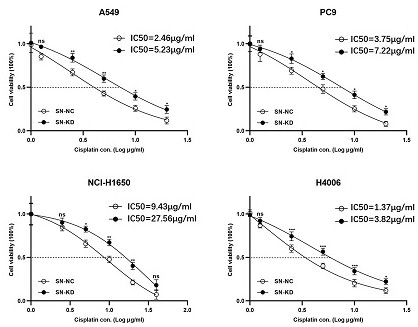
[0045] Example 1 Verify that the decreased expression of snoRNA SDORD33 leads to decreased sensitivity of lung cancer cells to cisplatin
[0046] First, four lung cancer cell lines A549, PC9, NCI-H1650, and H4006 were treated with different concentrations of cisplatin, and the snoRNA SNORD33 gene in these four lung cancer cell lines was knocked out. After 48 hours, the They were treated with different concentrations of cisplatin and tested by CCK-8 experiment. The results showed that after the snoRNA SNORD33 gene was knocked out, the expression of snoRNA SNORD33 in the four lung cancer cell lines was significantly reduced, the lethality of the cisplatin drug on the four lung cancer cell lines was significantly weakened, and the number of viable cells was significantly increased. The half-inhibitory concentration (IC50) was significantly increased ( figure 1 ), thus indicating that the downregulation of snoRNA SNORD33 expression is closely related to the sensitivity of lung ca...
Embodiment 2
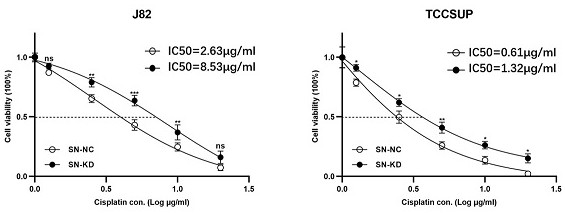
[0047] Example 2 Verification that the decreased expression of snoRNA SDORD33 leads to decreased sensitivity of bladder cancer cells to cisplatin
[0048] Firstly, two bladder cancer cell lines J82 and TCCSUP were treated with different concentrations of cisplatin, and the snoRNA SNORD33 gene in these two bladder cancer cell lines were respectively knocked out. After 48 hours, different concentrations of cisplatin Cisplatin drugs were treated separately and tested by CCK-8 experiment. The results showed that after the snoRNA SNORD33 gene was knocked out, the expression levels of snoRNA SNORD33 in the two bladder cancer cell lines were significantly reduced, the lethality of cisplatin on the two bladder cancer cell lines was significantly weakened, and the number of viable cells was significantly increased. The half-inhibitory concentration (IC50) of platinum drugs was significantly increased ( figure 2 ), thus indicating that the downregulation of snoRNA SNORD33 expression i...
Embodiment 3
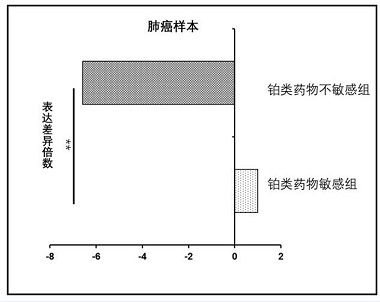
[0049] Example 3 Verify that there are significant differences in the expression of snoRNA SNORD33 in the plasma samples of lung cancer patients and bladder cancer patients with different curative effects on platinum-based drugs
[0050] After a lot of research, the inventor team found that there is a significant difference in the expression of snoRNA SNORD33 in the plasma samples of lung cancer patients and bladder cancer patients with different curative effects on platinum-based drugs, and snoRNA SNORD33 can be used as a marker for evaluating the efficacy of platinum-based drugs in patients with lung cancer and bladder cancer. molecular markers.
[0051] Plasma samples were collected from 57 lung cancer patients with good efficacy of platinum-based drugs and 62 lung cancer patients with poor efficacy of platinum-based drugs, plasma samples of 48 bladder cancer patients with good efficacy of platinum-based drugs and 59 patients with poor efficacy of platinum-based drugs. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com