Composition for detecting tumor residues of mother cell plasma cell-like dendritic cells and application of composition for detecting tumor residues of mother cell plasma cell-like dendritic cells
A composition and plasma cell technology, which can be used in measurement devices, biological tests, material inspection products, etc., can solve the problems of lack of detection methods and methods for the micro-residue of blastic plasmacytoid dendritic cell tumors, etc., and achieve a guaranteed accuracy rate. , Reduce missed detection and false detection, avoid the effect of missed detection or false detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
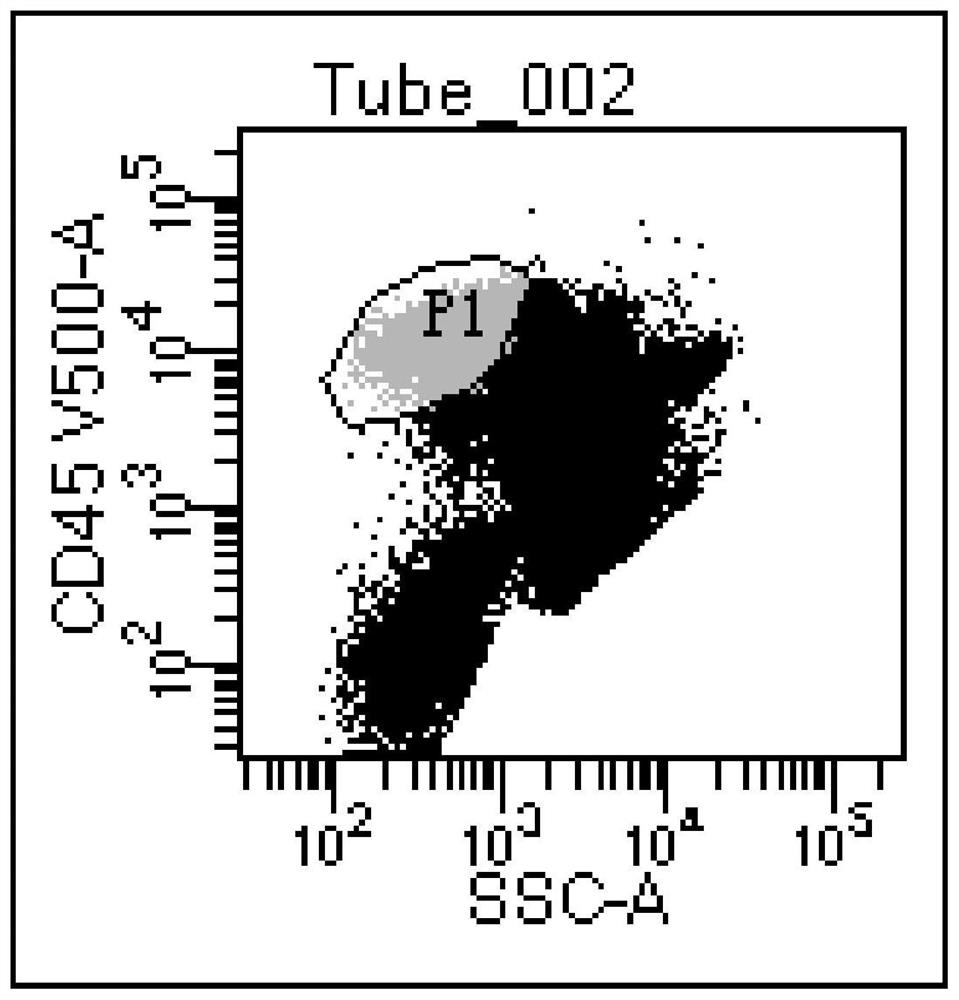
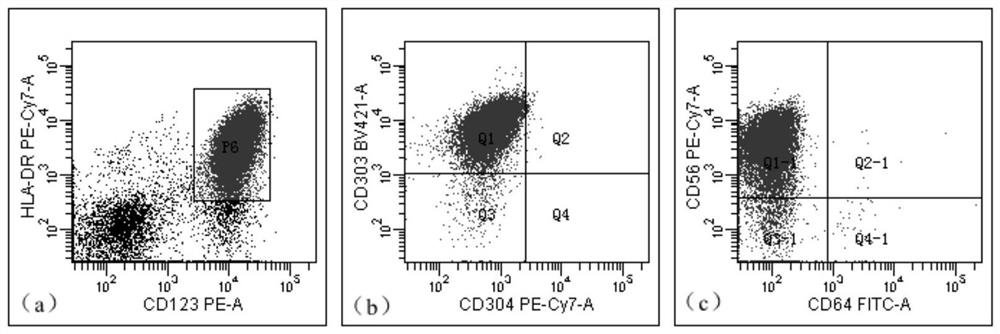
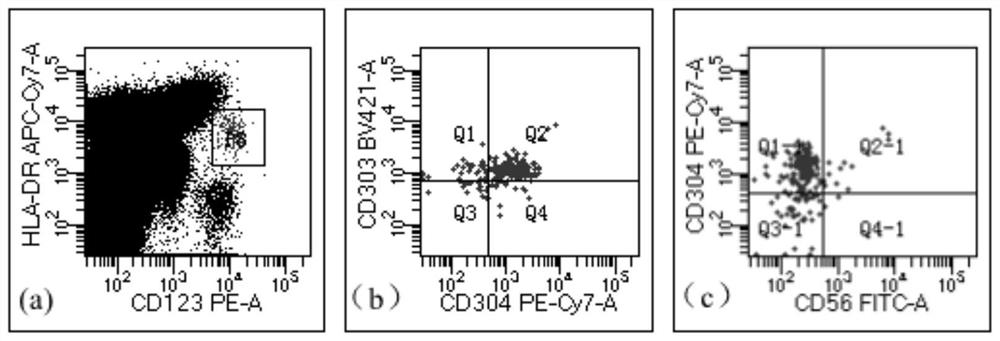
[0048] This embodiment provides a kit for detecting residual tumor of blastic plasmacytoid dendritic cells, including HLA-DRAPC-cy7, CD4 APC, CD11b PE-cy7, CD15 BV450, CD56 FITC, CD64 FITC, CD123 PE, CD303BV421 , CD304 PE-cy7, CD34 PerCP, CD33APC and CD45 V500 antibodies, erythrocyte lysate, PBS buffer, wherein, composition one includes CD56 FITC, CD123 PE, CD34 PerCP, CD4 APC, CD304 PE-cy7, HLA-DRAPC- cy7, CD303 BV421 and CD45 V500 antibodies, composition 2 includes CD64 FITC, CD123 PE, CD33 APC, CD11b PE-cy7, HLA-DRAPC-cy7, CD15 BV450 and CD45 V500 antibodies.
[0049] Red blood cell lysate was purchased from BD Company, including the following components: ammonium chloride, potassium dihydrogen phosphate, disodium edetate and paraformaldehyde;
[0050] The concentration of PBS buffer (phosphate buffer) is 0.01M, the pH is 7.2-7.4, including the following components: potassium dihydrogen phosphate 1.8mmol / L, disodium hydrogen phosphate 10mmol / L, sodium chloride 137mmol / L and...
Embodiment 2
[0053] The difference between this example and Example 1 is that in composition one, the mass ratio of CD56 FITC, CD123 PE, CD34 PerCP, CD4 APC, CD304 PE-cy7, HLA-DR APC-cy7, CD303 BV421 and CD45 V500 antibodies is 12:24:100:3.6:60:30:30:80, the rest of the conditions are consistent with Example 1.
experiment example 1
[0059] The kit in Example 1 was used to detect and analyze the samples of patients with blastic plasmacytoid dendritic cell tumor and the samples of normal test subjects, and the detection steps were as follows:
[0060] S1. Add 23 μL of Composition 1 and 18 μL of Composition 2 into two different test tubes, and then add 200 μL of the sample to be tested in both test tubes (adjust the cell solubility to 10 7 / mL), incubate in the dark for 15min;
[0061] S2. Add 1 mL of erythrocyte lysate to each of the two test tubes, mix evenly, and then stand in the dark for 8 minutes at room temperature to obtain a lysate mixture;
[0062] S3. Centrifuge the lysis mixture at 300 rct / min for 5 minutes, remove the supernatant, and obtain a lysis precipitate;
[0063] Step S4, add 1mL PBS to the lysed precipitate and mix evenly, then centrifuge at 150rct / min for 5min, then add 150μL PBS to resuspend to obtain a resuspension solution;
[0064] S5, detect the resuspended solution on the flow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com