Method for evaluating ripdicaine emulsifiable paste
A technology for risprocaine and cream, which is applied in the field of evaluating samples developed for risprocaine cream, and can solve problems such as the inability to accurately evaluate the similarity and difference in results of transdermal experiments between self-developed samples and reference preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Basic experiment example 1 Preparation of self-developed samples
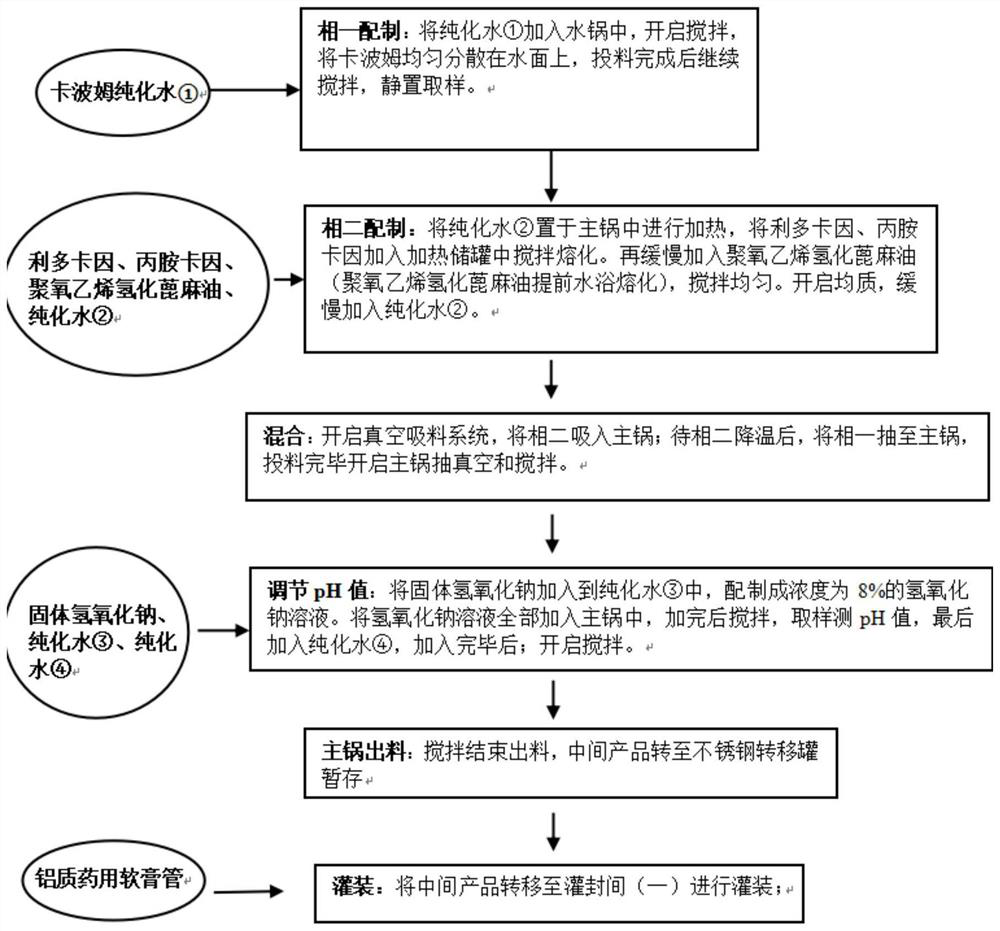
[0047] The self-developed sample Liprocaine Cream is a local anesthetic and analgesic preparation for external use. It is suitable for all kinds of needle puncture and surgical anesthesia for superficial skin and mucous membranes. Each g contains lidocaine 25mg and prilocaine 25mg. The prescribing information is as follows:
[0048] materials effect Content (mg / g) Lidocaine active ingredient 25 Prilocaine active ingredient 25 carbomer thickener 8.0 Polyoxyethylene Hydrogenated Castor Oil Emulsifier 19.0 sodium hydroxide pH regulator 4.08 purified water solvent 918.92
[0049] Process flow chart such as figure 1 .
Embodiment 1
[0050] Embodiment 1 A kind of evaluation method of Liprocaine emulsifiable cream
[0051] The reference preparation in this example is EMLA cream, purchased from Aspen Pharma Trading Limited, Sweden, registration number H20100154.
[0052] (1) Preparation of mobile phase:
[0053] Weigh 3.03g of potassium dihydrogen phosphate and dissolve it in 700mL of water, adjust to pH 7.20±0.05 with 5mol / L sodium hydroxide, filter with 0.22μm water filter, take 630mL of filtrate, dilute to 1000mL with acetonitrile, shake Uniform, ultrasonic degassing, that is.
[0054] Diluent (receiving medium): 0.9% NaCl solution.
[0055]Reference substance solution preparation: take 20 mg of prilocaine reference substance and 20 mg of lidocaine reference substance respectively, accurately weigh them, put them in a 100mL measuring bottle, add diluent to dissolve and dilute to the mark, and shake well.
[0056] (2) The Franz vertical diffusion device was used in the transdermal experiment (the effect...
Embodiment 2
[0077] Embodiment 2 pigskin repeated utilization is to the transdermal experiment of Liprocaine emulsifiable cream
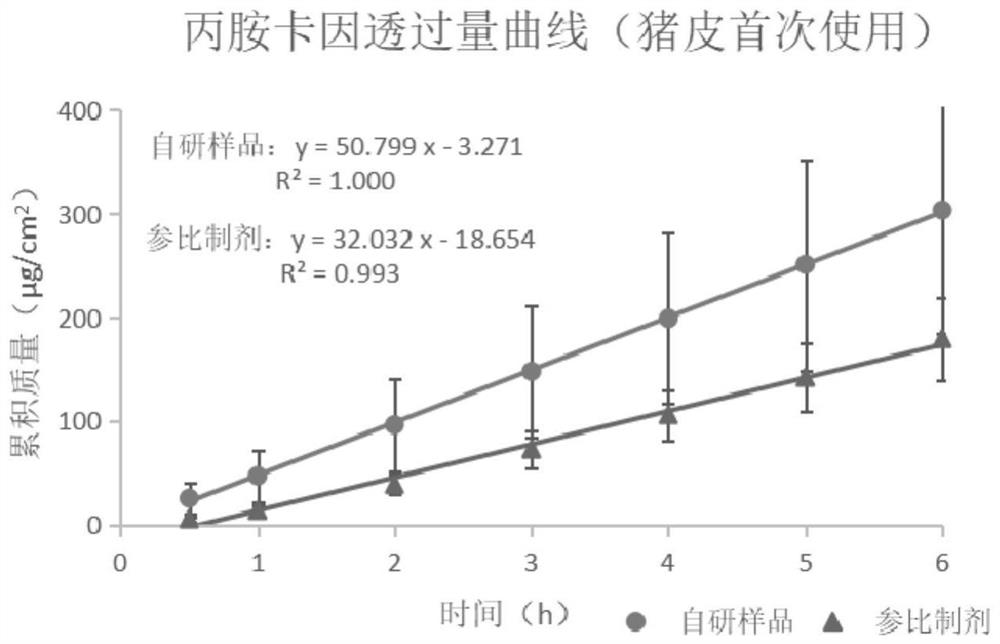
[0078] Get 6 pieces of pigskin, take the self-developed sample as the object of investigation, repeat the transdermal experiment 4 times (with reference to the initial transdermal experiment in Example 1), the results are shown in Figure 9-10 . Analyzing the 4 results, the ratio of the transdermal rate of prilocaine between the results of the subsequent three and the initial experiment was between 0.90-0.95, and the ratio of transdermal rate of lidocaine was 0.91-0.95.
[0079] In addition, 6 pieces of pigskin were taken, and the self-developed samples and the reference preparation were used as the investigation objects. Comparing preparations, comparing cross-loading, and repeated use of pigskin on the sample penetration rate, the results are shown in Figure 11-12 . The results showed that the ratio of prilocaine transdermal rate of the two self-developed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com