Sugar-modified metal helical complex, preparation method thereof and application of complex as amyloid protein degradation device
A technology of amyloid and helical coordination, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of difficult phagocytosis, phagocytic cell damage, lysosome rupture, etc., and achieve the effect of stable physiological conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A method for preparing a sugar-modified metal helical complex of the present invention specifically comprises the following steps:
[0032] Anhydrous FeCl 2 , 5-(prop-2-yn-1-yloxy)pyridinecarbaldehyde and 2-(2,2'-bipyridine-5-oxygen)-1-methylbenzylamine were dissolved in anhydrous methanol at room temperature After stirring for 36-72h (preferably 48h), cool naturally to room temperature, filter through a diatomaceous earth plug, and remove the solvent under vacuum to obtain the alkylated metal helical complex (purple solid product); dissolve the alkylated metal helical complex In methanol, add cuprous iodide and sugar azide, heat and reflux at 50-70°C (preferably 65°C) under nitrogen protection for 12-24h (preferably 18h); remove the copper salt by filtration to obtain a sugar-modified metal helical complex thing.
[0033] Wherein, the structural formula of the 5-(prop-2-yn-1-yloxy)pyridinecarbaldehyde is as follows:
[0034]
[0035] Preferably, the 2-(2,2'-bipyr...
Embodiment 1
[0057] The preparation of the metal helical complex of embodiment 1 sugar modification
[0058] 2.5 mg / mL of anhydrous FeCl 2 (1 equivalent), 4.5 mg / mL of 5-(prop-2-yn-1-yloxy)pyridinecarbaldehyde (1.5 equivalents), 9.5 mg / mL of R-2-(2,2'-bipyridine- 5-Oxo)-1-methylbenzylamine (1.5 equivalents) was dissolved in 10 mL of anhydrous methanol, stirred at room temperature for 48 h, the reaction mixture was naturally cooled to room temperature, filtered through a diatomaceous earth plug, and the solvent was removed under vacuum to obtain The purple solid product is the metal alkynylation helical complex; then the alkynylated metal helical complex (1 equivalent) is dissolved in methanol, cuprous iodide (0.1 equivalent) and azide sugar (4.5 equivalents) are added, nitrogen protection Heat and reflux at 65°C for 18 hours; after the reaction, remove the copper salt by filtration to obtain a sugar-modified metal helical complex with a structure of Λ1, and the structural formula is as fo...
Embodiment 2
[0067] Example 2 Application of sugar-modified metal helical complexes as amyloid degraders in inhibiting hIAPP aggregation
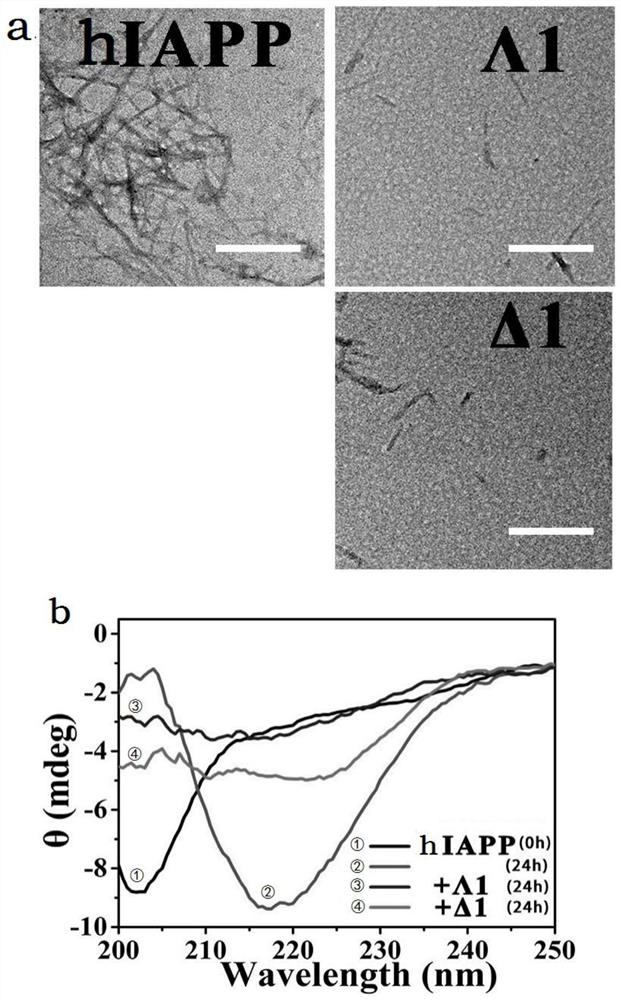
[0068] First, lyophilized hIAPP powder was dispersed in hexafluoroisopropanol and stored at -20°C. The dispersion was dried under a nitrogen atmosphere, the hIAPP peptide was redissolved in ultrapure water, with or without the sugar-modified metal helical complex prepared in Example 1, and incubated at 37°C for 24 hours. The effects of sugar-modified metal helical complexes on the aggregation morphology of hIAPP were examined by transmission electron microscopy.
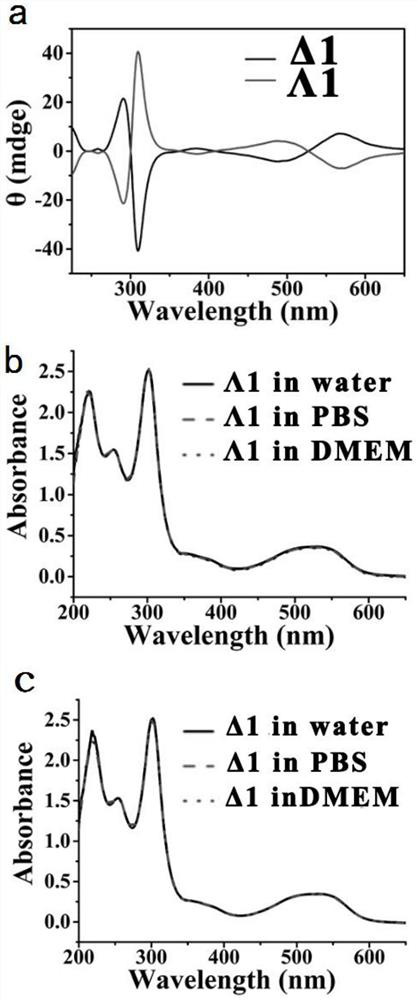
[0069] Such as figure 2 As shown in a, both Δ1 and Δ1 significantly inhibited the abnormal aggregation of proteins. The effects of sugar-modified metal helical complexes on hIAPP aggregation were further confirmed by circular dichroism, as shown in figure 2 As shown in middle b, hIAPP alone transformed from α-helical structure (characteristic peak 205nm) to protein aggregates with β-sheet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com