Granules of soy protein
A granulation and protein technology, applied in plant protein processing, food science, food forming, etc., can solve the problems of poor sedimentation, difficulty in improving solubility, and insufficient improvement of soybean protein water solubility, etc., to achieve insoluble residue Less, easy to drink effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0122]Hereinafter, although an Example and a comparative example demonstrate this invention further, this invention is not limited to the following example.
[0123] 〔test methods〕
[0124] The measurement method used in this example will be described below.
[0125] [Average particle size, uniformity U, content ratio of coarse powder and fine powder]
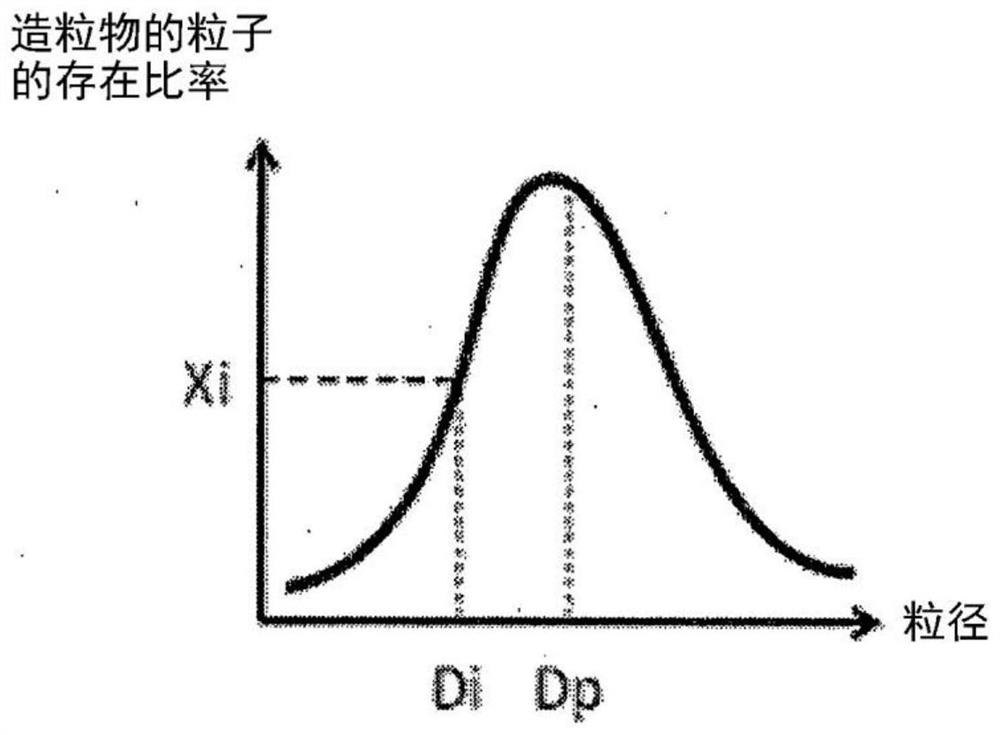
[0126] Using the laser diffraction / scattering type particle size distribution analyzer Mastersizer 3000 (manufactured by Malvern Co.) and the accompanying software Mastersizer 3000, the volume-based particle size distribution plotted by plotting the particle diameter on the horizontal axis and the abundance ratio of particles on the vertical axis was obtained. . As the measurement conditions, the hopper gap is 3.5 mm, the feeder strength is 20-40%, and the air pressure for powder conveying is 0.2 bar.
[0127] From the obtained volume-based particle size distribution, the average particle size (median size), the content rati...
manufacture example 1
[0131] Soybean protein-containing composition 1 before granulation was prepared by mixing the following components.
[0132] Refined soybean protein (trade name HD101R, manufactured by FUJI OIL CO.,LTD.)
[0133] Maltodextrin
[0134] cocoa powder
[0135] salt
[0136] sweetener
[0137] Vitamin Blend (contains vitamins A, B1, B2, B6, B12, C, D, E, K, pantothenic acid, folic acid, niacin)
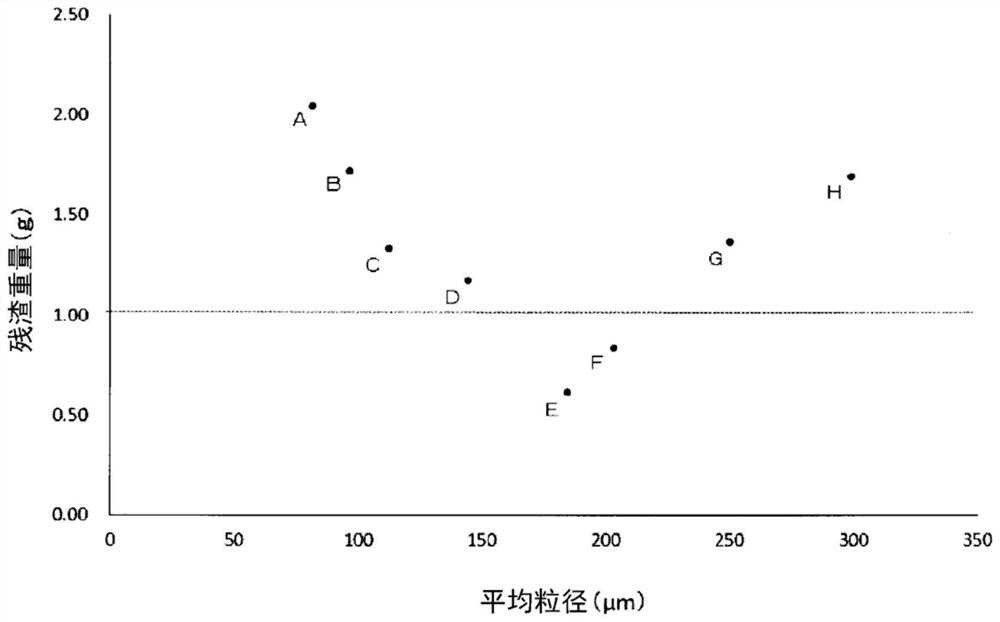
[0138] The prepared above-mentioned composition 1 was introduced into a fluidized bed machine together with an emulsifier (polyglyceryl fatty acid ester) as a liquid component (binder) and an aqueous solution of thickening polysaccharides (pullulan and gum arabic). In the granulator, granules having the composition shown in Table 1 were obtained. In addition, eight types of granulated materials A to H shown in Table 2 were obtained by changing the binder addition amount, spray air flow rate, etc.
[0139] The particle size of the composition 1 before granulation was 80 to 100 μm, and...
manufacture example 2
[0151] In Production Example 2, the average particle diameter was substantially the same, and the difference in residue weight based on the uniformity U was confirmed.
[0152] The above-mentioned composition 1 prepared in Production Example 1 was introduced into a fluidized bed granulator together with an emulsifier as a liquid component (binder) and a thickened polysaccharide aqueous solution. Air flow rate etc., thereby can obtain 4 kinds of granules (I~L) shown in Table 3.
[0153] Moreover, similarly to manufacture example 1, the evaluation of water solubility was performed about the obtained granulated material I-L.
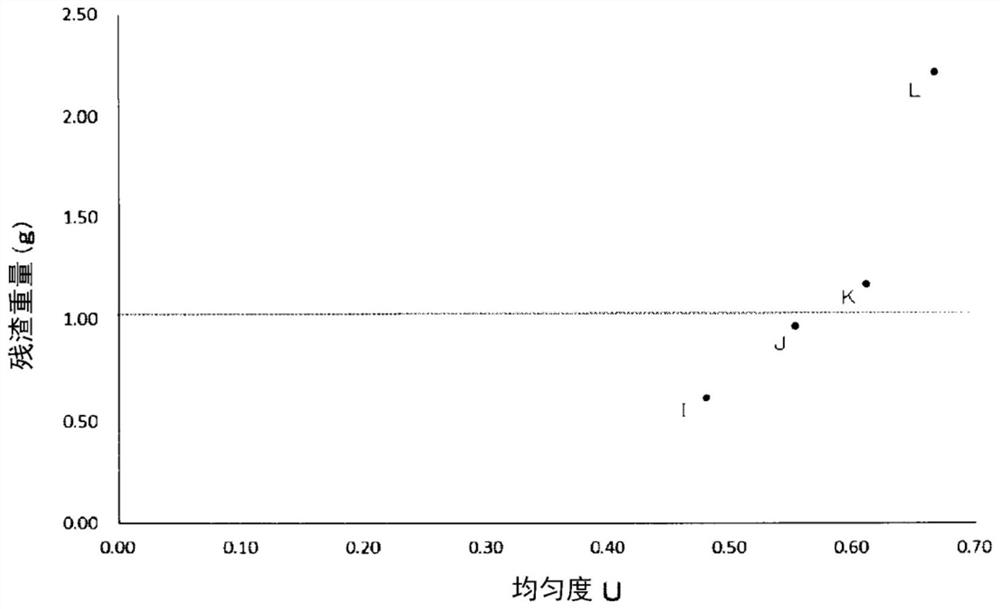
[0154] The results are shown in the following Table 3 and image 3 .
[0155] [table 3]
[0156] Table 3 Manufacturing Example 2
[0157]
[0158] From Table 3 and image 3 As a result, it was found that among the granules with an average particle diameter in the range of 150 to 220 μm, the granules I and J with a uniformity U of 0.58 or less had a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com