Synthesis process of 3-aminopyridine
A synthesis process and aminopyridine technology, applied in the field of synthesis technology of 3-aminopyridine, can solve the problems of high cost, troublesome waste water treatment, unsuitable for industrial mass production and application, etc., and achieve high yield, simple preparation steps, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthetic technique of 3-aminopyridine comprises the following synthetic steps:
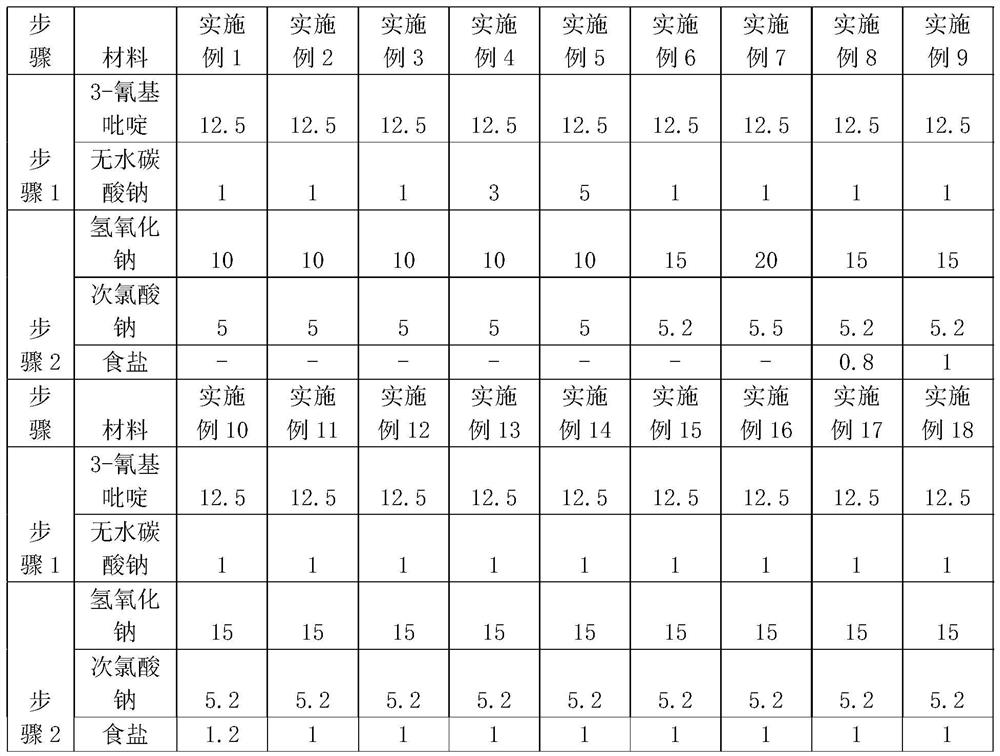
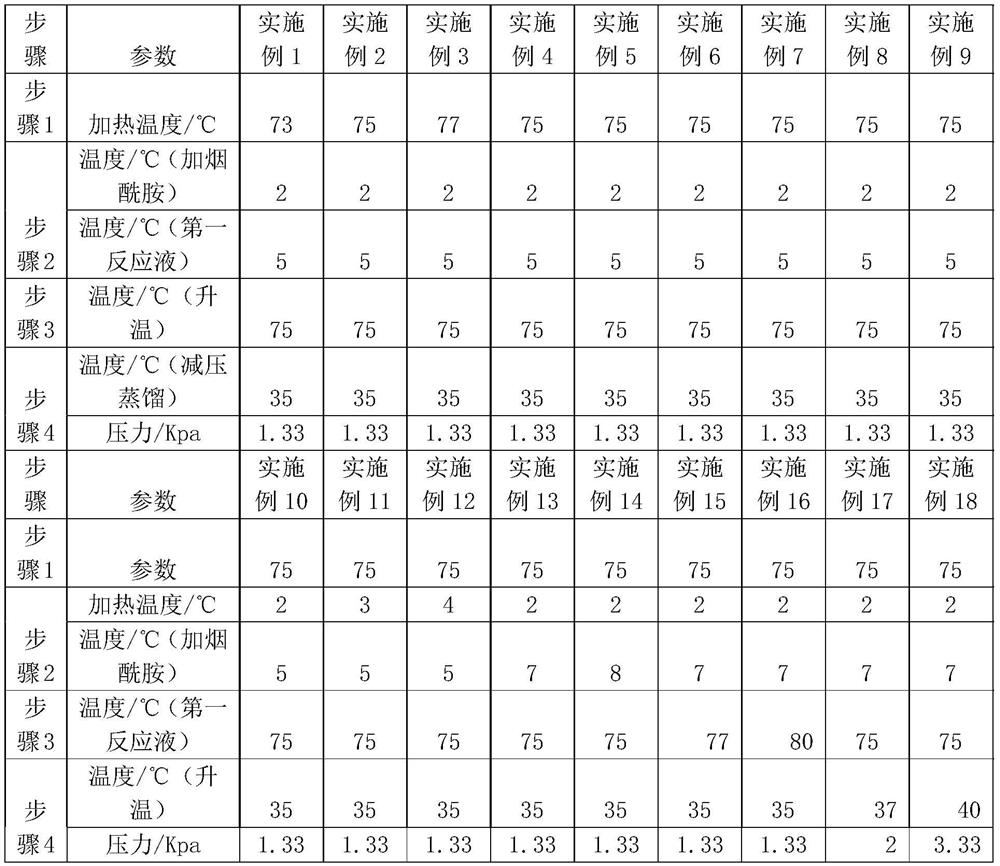
[0034] Step 1: Preparation of nicotinamide: The device for nicotinamide preparation uses a reflux device, specifically adding 60ml of water to a three-necked beaker, and then adding 12.5g of 3-cyanopyridine and 1g of anhydrous sodium carbonate in sequence, stirring while reacting, and Slowly heat up to 73°C, and then keep warm for about 6 hours until the end of the reaction. After reaching the end of the reaction, cool to room temperature and stop stirring to obtain a reaction solution containing nicotinamide for later use.
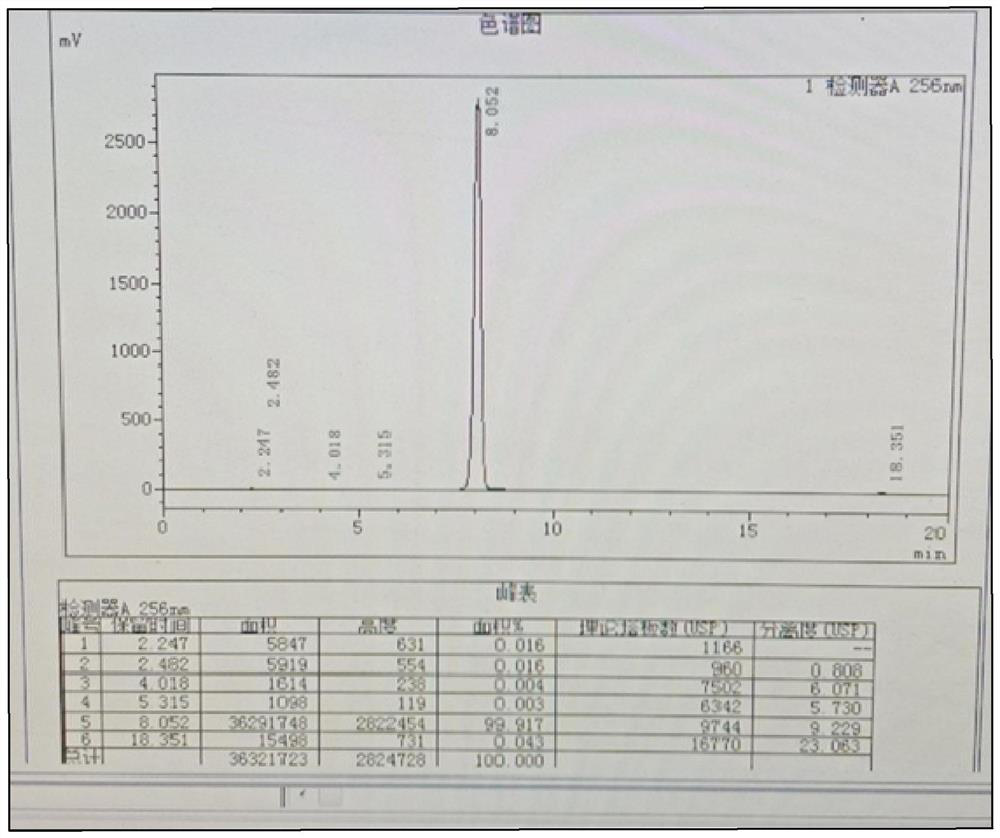
[0035] During the preparation of nicotinamide, when determining the end point of the reaction, high-performance liquid chromatography is used for detection. The chromatographic conditions are as follows: chromatographic column: C18, 300mm*3.9mm, 5μm; detection wavelength: 254nm; mobile phase: methanol: 0.005mol / L sodium heptanesulfonate=30:70; flow rate: 1ml / min....
Embodiment 2
[0043] The synthetic technique of 3-aminopyridine comprises the following synthetic steps:
[0044] Step 1: Preparation of nicotinamide: The device for nicotinamide preparation uses a reflux device. Add 60ml of water into a three-necked beaker, then add 12.5g of 3-aminopyridine and 1g of anhydrous sodium carbonate in sequence, stir while reacting, and slowly heat up to 75°C, and then keep warm for about 6 hours until the end of the reaction. After reaching the end of the reaction, cool to room temperature, stop stirring, and obtain a reaction solution containing nicotinamide for later use.
[0045] The judgment of the reaction end point during the preparation of nicotinamide is the same as in Example 1;
[0046] Step 2: Add the nicotinamide-containing reaction liquid in step 1 into the sodium hydroxide solution, and keep the temperature at 2°C during the addition; then add the sodium hypochlorite solution and stir to obtain the first reaction liquid, and control the temperatur...
Embodiment 3
[0052] The synthetic technique of 3-aminopyridine comprises the following synthetic steps:
[0053]Step 1: Preparation of nicotinamide: The equipment for nicotinamide preparation uses a reflux device. Add 60ml of water into a three-necked beaker, and then add 12.5g of 3-aminopyridine and 1g of anhydrous sodium carbonate in sequence, stir while reacting, and slowly heat up to 77°C, and then keep warm for about 6 hours until the end of the reaction. After reaching the end of the reaction, cool to room temperature, stop stirring, and obtain a reaction solution containing nicotinamide for later use.
[0054] The judgment of the reaction end point during the preparation of nicotinamide is the same as in Example 1;
[0055] Step 2: Add the nicotinamide-containing reaction liquid in step 1 into the sodium hydroxide solution, and keep the temperature at 2°C during the addition process; then add the sodium hypochlorite solution and stir to obtain the first reaction liquid, and control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com