Cyclic carbonate nucleoside compound and application thereof
A technology of compounds and nucleosides, applied in the field of medicine, can solve the problems of sows with greatly fluctuating morbidity, no treatment, and easy development of infectious peritonitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
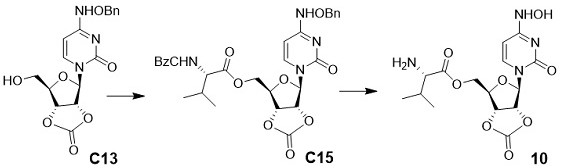
[0057] Embodiment 1: the preparation of compound 1
[0058]
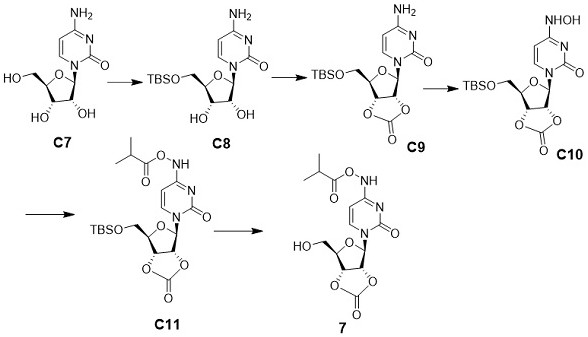
[0059] Take a dry 500.0 mL flask, dissolve GS-441524 (10.00 g, 34.33 mmol, 1.0 eq), imidazole (7.01 g, 103.00 mmol, 3.0 eq) in 50.0 mL of anhydrous DMF. Slowly add TBSCl (6.21g, 41.20 mmol, 1.2 eq) at 0°C, stir for 1 h, TLC (DCM : MeOH = 4:1) detects that the reaction of raw materials is complete, dilute with ethyl acetate (200 mL), wash with water (200 mL×2), washed with saturated brine (200 mL×1), collected the organic phase, added anhydrous sodium sulfate to dry, and distilled off the organic phase under reduced pressure to obtain a crude product, which was separated and purified by silica gel column chromatography (methanol:dichloro Methane=1:20) to obtain white solid compound C1 (methanol:dichloromethane=1:20, 12.53 g, 90%). 1 H NMR (400 MHz, CD 3 OD) δ 7.88 (s, 1H), 7.00 – 6.80 (m, 2H), 4.79 (d, J = 4.8 Hz, 1H), 4.30 –4.12 (m, 2H), 3.94 (dd, J = 11.6, 2.6 Hz, 1H), 3.82 (dd, J = 11.7, 3.3 Hz,1H), 3.3...
Embodiment 2
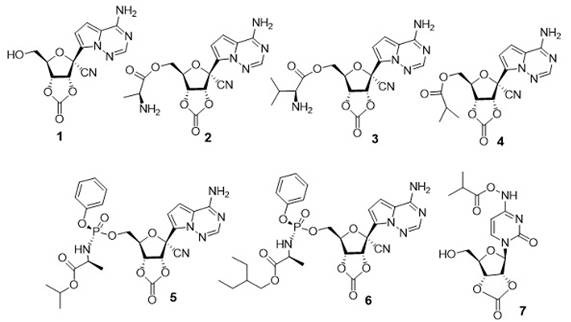
[0062] Embodiment 2: the preparation of compound 2
[0063]
[0064] Take a dry 100.0 mL flask, add compound 1 (1.20 g, 3.79 mmol, 1.0 eq), L-Boc-alanine (0.72 g, 3.79 mmol, 1.0 eq), DCC (0.78 g, 3.79 mmol, 1.0 eq ) and a catalytic amount of DMAP were dissolved in 20.0 mL of anhydrous DMF and reacted for 12 h. TLC (DCM:MeOH=10:1) detected that the reaction of raw materials was complete, diluted with ethyl acetate (60 mL), washed with water (20 mL×2), washed with saturated brine (20 mL×1), collected the organic phase, Add anhydrous sodium sulfate to dry, and then spin-dry under reduced pressure at 40°C. Silica gel column chromatography shows white solid C4 (DCM:MeOH = 100:3, 0.91 g, 49%). 1 H NMR (400 MHz, CDCl 3 ) δ 7.96 (s, 1H), 7.07 (d, J = 4.6 Hz, 1H), 6.71 (d, J = 4.1 Hz, 1H), 5.37 – 5.29 (m, 1H), 5.05 (d, J = 6.9 Hz, 2H), 4.63 (q, J =4.8 Hz, 2H), 4.59 – 4.43 (m, 2H), 4.37 – 4.27 (m, 1H), 1.43 (s, 7H), 13 C NMR (101 MHz, CDCl 3 ) δ 176.46, 171.83, 155.38, 15...
Embodiment 3
[0066] Embodiment 3: the preparation of compound 3
[0067]
[0068] Take a dry 100.0 mL flask, add compound 1 (1.20 g, 3.79 mmol, 1.0 eq), Boc-valine (0.82 g, 3.79 mmol, 1.0 eq), DCC (0.78 g, 3.79 mmol, 1.0 eq) and A catalytic amount of DMAP was dissolved in 20.0 mL of anhydrous DMF and reacted for 12 h. TLC (DCM:MeOH=10:1) detected that the reaction of raw materials was complete, diluted with ethyl acetate (60 mL), washed with water (20 mL×2), washed with saturated brine (20 mL×1), collected the organic phase, Add anhydrous sodium sulfate for drying, and then spin-dry under reduced pressure at 40°C. Silica gel column chromatography shows white solid C5 (DCM:MeOH=100:3, 0.80 g, 42%). 1 H NMR (400 MHz, CDCl 3 ) δ = 8.02 (s, 1H), 7.07 (d, J =4.7, 1H), 6.78 (d, J =4.6, 1H), 6.34 (s, 2H), 5.98 (d, J =8.0, 1H), 5.33 (dd, J =5.2, 8.0, 1H), 5.05(d, J =9.1, 1H), 4.63 (q, J =5.1, 1H), 4.50 (qd, J =5.2, 12.0, 2H), 4.24 (dd, J =4.7, 8.9, 1H), 2.15 (m, 1H), 1.43 (d, J =2.6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com