Mesenchymal stem cell with immunosuppression and anti-inflammatory functions, and preparation method and application thereof
A stem cell, immunosuppressive technology, applied in biochemical equipment and methods, anti-inflammatory agents, animal cells, etc., can solve the problems of inability to inhibit T cell proliferation, not exerting anti-inflammatory effect as expected, and achieve excellent therapeutic effects, Simple preparation method and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
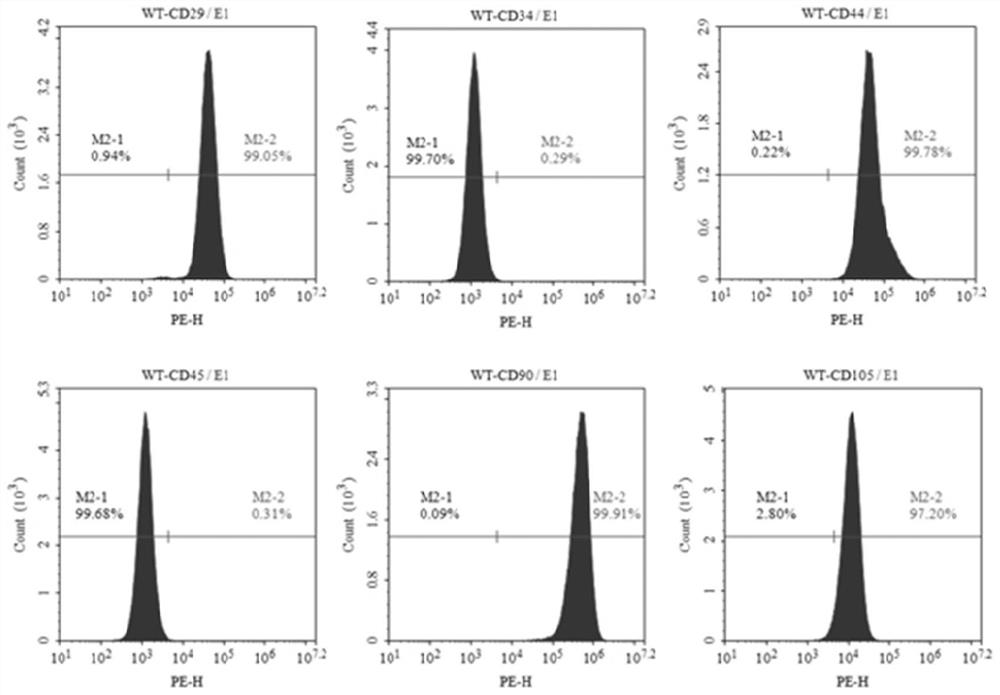
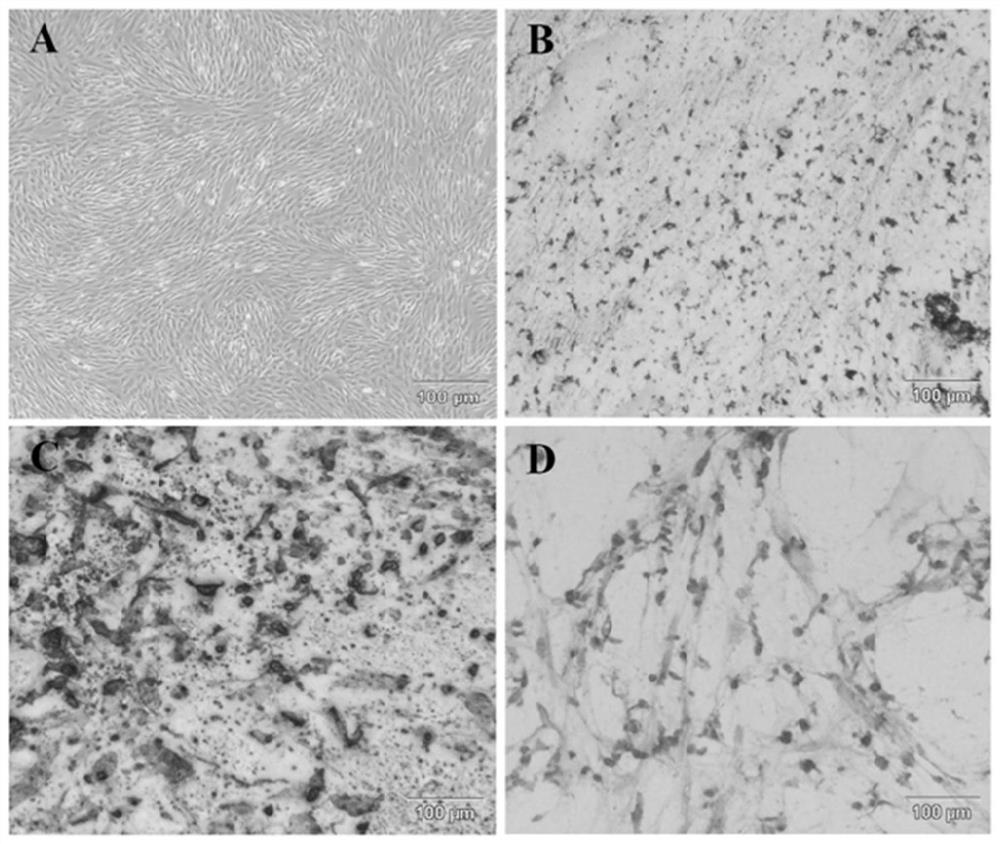
[0033] Example 1 Isolation, cultivation and identification of human umbilical cord mesenchymal stem cells
[0034] Using existing technology to isolate, culture and identify human umbilical cord mesenchymal stem cells from neonatal umbilical cord, the specific steps are:
[0035] 1. Isolation and culture of MSCs
[0036] Isolation and culture of human umbilical cord mesenchymal stem cells (hUC-MSCs): Fresh umbilical cord samples collected under sterile conditions (the samples were from healthy puerperas who were negative for infectious diseases such as hepatitis B, hepatitis C, HIV and syphilis and had no genetic diseases) in Wash the ultra-clean workbench with normal saline to remove surface debris, then soak it in alcohol for 1 minute, then transfer it to clean normal saline to wash off the alcohol on the umbilical cord; then cut off both ends of the umbilical cord and remove the umbilical cord as much as possible coagulated blood, and cut off about 2cm of umbilical cord; p...
Embodiment 2
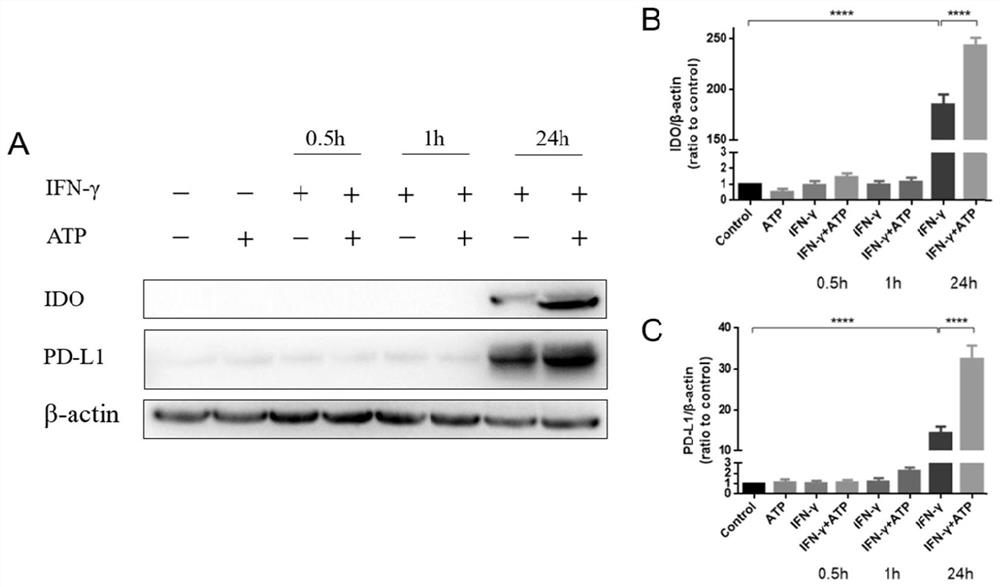
[0045] Example 2 Induction, culture and identification of human umbilical cord mesenchymal stem cells with enhanced immunosuppressive or anti-inflammatory functions
[0046] On the basis of Example 1, hUC-MSCs were jointly induced by ATP and IFN-γ to obtain hUC-MSCs with enhanced immunosuppressive or anti-inflammatory functions, and the specific steps were:
[0047] 1. Preparation of hUC-MSCs with enhanced immunosuppressive or anti-inflammatory function induced in vitro
[0048] The hUC-MSCs cryopreserved cells obtained in Example 1 were resuscitated, and 4×10 4 cells / cm 2 The cell density was seeded at 75cm 2 Place the culture bottle in an incubator (37°C, 5% CO 2 incubator), when the cultured cells grew to a cell density >80%, they were digested and dissociated with TrypLE and subcultured, and the digested cells were divided into 4×10 4 cells / cm 2 The cell density was seeded at 75cm 2 culture flasks, and divide the cells into ATP and IFN-γ single and joint treatment gr...
Embodiment 3
[0097] Example 3 Application of Human Umbilical Cord Mesenchymal Stem Cells with Enhanced Immunosuppressive or Anti-inflammatory Function in the Treatment of Collagen-Induced Arthritis Mice
[0098] 1. Establishment and evaluation of collagen induced a4th4itis (CIA) model
[0099] ⑴Preparation of collagen-induced emulsion: Use a homogenizer (5mm or less in diameter) or a stirrer to emulsify 4mg / ml Complete Freund's Adjuvant (CFA) and collagen solution in a three-way tube sealed syringe (use The same is true for booster injections of incomplete Ford's adjuvant IFA). Since heating denatures collagen, which does not induce arthritis, the syringe is clamped to a ring holder and kept cold by immersing it in an ice-water bath.
[0100] (2) Add a maximum of 2.5ml of CFA to the syringe, and then slowly add an equal volume of collagen solution (2mg / ml collagen dissolved in 0.05M acetic acid), and stir slowly (1000-3000rpm) while adding. Note: To ensure a high quality emulsion, the ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com