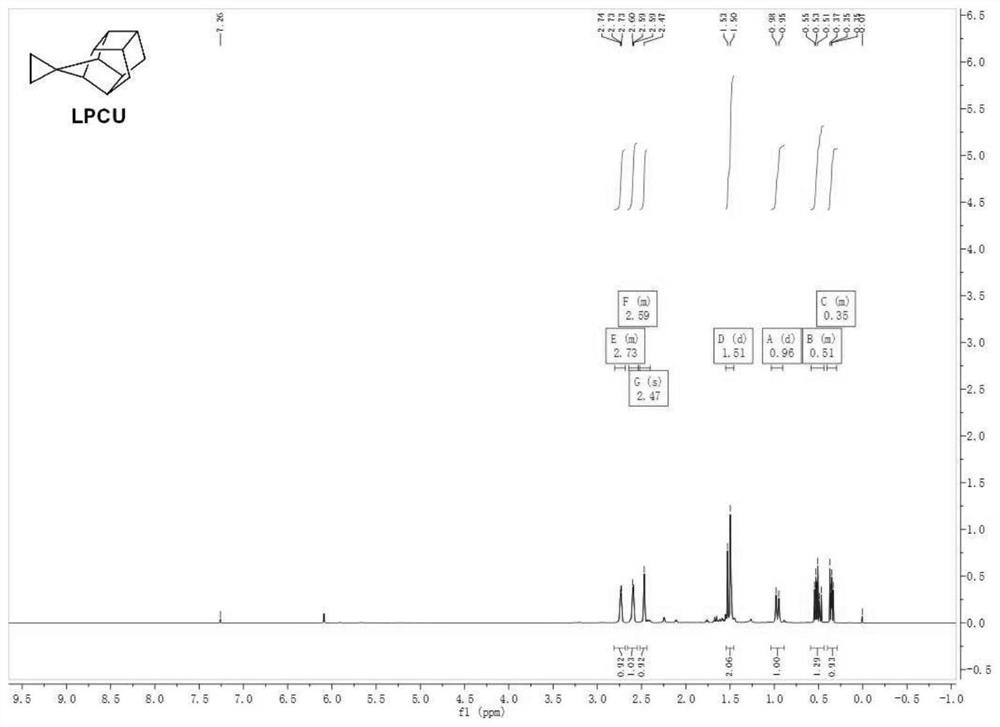
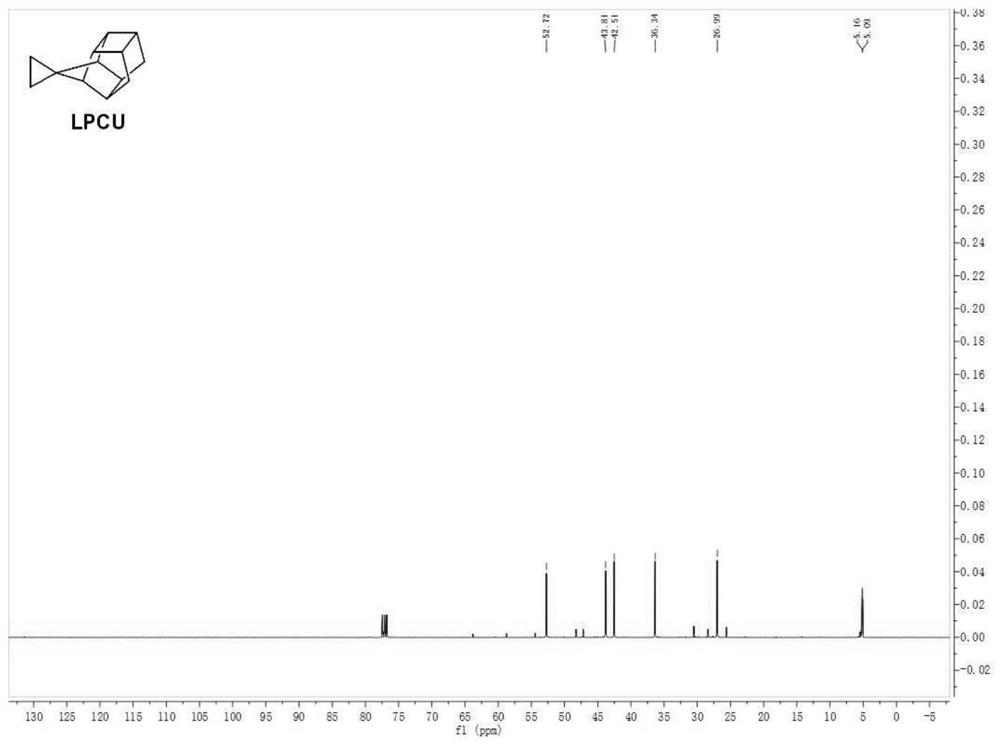
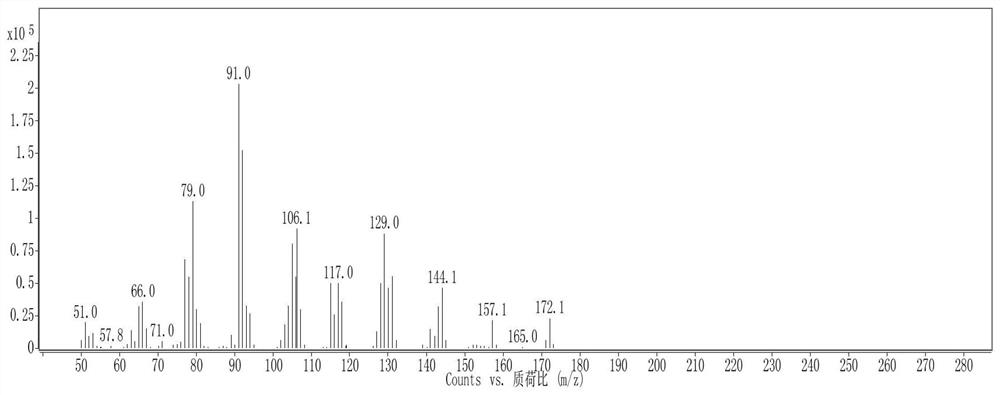
Cage hydrocarbon with spiral ring structure as well as preparation method and application of cage hydrocarbon
A spiro-ring structure, cage-like technology, applied in the field of cage-like hydrocarbons and their preparation, can solve the problems of limited application and limited fuel solubility, and achieve the effects of large mass density and volume calorific value, increasing added value and increasing energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of cyclopentadiene:
[0063] Depolymerization reaction of dicyclopentadiene: Add 100ml of dicyclopentadiene liquid into a 250ml single-necked round-bottom flask, set up a rectification device, heat the reaction device to 180°C with a heating mantle, and heat to reflux for 25 minutes, when the distillation head When the thermometer at the branch end showed a reading of 42°C, distillate flowed out from the tail joint at this time, and the distillate was a colorless liquid with a gasoline smell, which was monocyclopentadiene. In order to avoid the re-polymerization of the obtained cyclopentadiene monomer at room temperature, the receiving device should be protected at low temperature with an ice-salt bath. When the depolymerization time reaches 1 hour, no distillate flows out from the tail joint, and a total of 70ml of cyclopentadiene monomer is depolymerized, and the depolymerization rate is 70%. The newly prepared cyclopentadiene monomer can be frozen Save f...
Embodiment 2
[0065] Synthetic method 1 of spirocyclization cyclopentadiene:
[0066] A 50% aqueous NaOH solution (1.2 L, 10 equiv), 2,6-di-tert-butyl-p-cresol (20 mg) and methyltributylammonium chloride (75% aqueous solution, 5.35 g , 0.007 equivalent). Then freshly prepared cyclopentadiene (150 g, 2.27 mol, 1 equiv) was added to the above solution at 26 °C. The mixture was stirred at 26°C, and 1,2-dichloroethane (225 g, 1 equivalent) was slowly added dropwise to the above mixture (over 75 min). After the dropwise addition was completed, the stirring reaction was continued at 26° C. for 1 h. After the reaction was completed, the product spiro three-membered cyclocyclopentadiene was distilled off under reduced pressure. Finally, 170 g of product were obtained with a yield of 81%.
[0067] The synthesis of spiro four-membered cyclocyclopentadiene and spiro five-membered cyclopentadiene only needs to replace the raw material 1,2-dichloroethane with 1,3-dichloropropane and 1,4-dichlorobuta...
Embodiment 3
[0069] Synthetic method 2 of spirocyclic cyclopentadiene:
[0070] 120 ml of tetrahydrofuran was added into a double-necked round bottom flask at 0°C as a reaction solvent, and then sodium hydride (18.18 g, 0.45 mol, 2.6 equivalents) was added to the above solvent. Freshly prepared cyclopentadiene (17.42 ml, 0.21 mol, 1 equiv) was slowly added dropwise to the above solution (over 1 h) at 0°C. After the dropwise addition was completed, it was raised to room temperature and continued to stir for 20 minutes, the solution turned pink, then cooled to 0°C, and then 1,2-dichloroethane (13.78ml, 0.17mol, 1 equivalent) was slowly added dropwise To the above mixture (more than 60min). After the dropwise addition was completed, the mixture was raised to room temperature and continued to stir for 12 h. After the reaction, under the condition of 0°C, water-containing tetrahydrofuran was slowly added to the reaction solution to remove excess unreacted sodium hydride. Then add 80ml of wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com