Active compound and application thereof in protection of side-chain carboxyl compound
A compound and chain carboxyl technology, which is applied in the field of protection of biochemical products and side chain carboxyl compounds, can solve the problem of inability to obtain amino acids with high chiral purity, and achieve the effect of high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] A kind of synthetic method of active compound, the step of synthesizing active compound comprises:
[0105] Step 1: In a 10L reaction flask equipped with a thermometer, add N,N-diisopropylcarbodiimide 1.75kg, 3-methyl-3-pentanol 2.8kg, and cuprous chloride 420g;
[0106] Step 2: Stir the reaction at 25°C for 3.5 days;
[0107] Step 3: Suction filtration to obtain 4.5 kg of active compound.
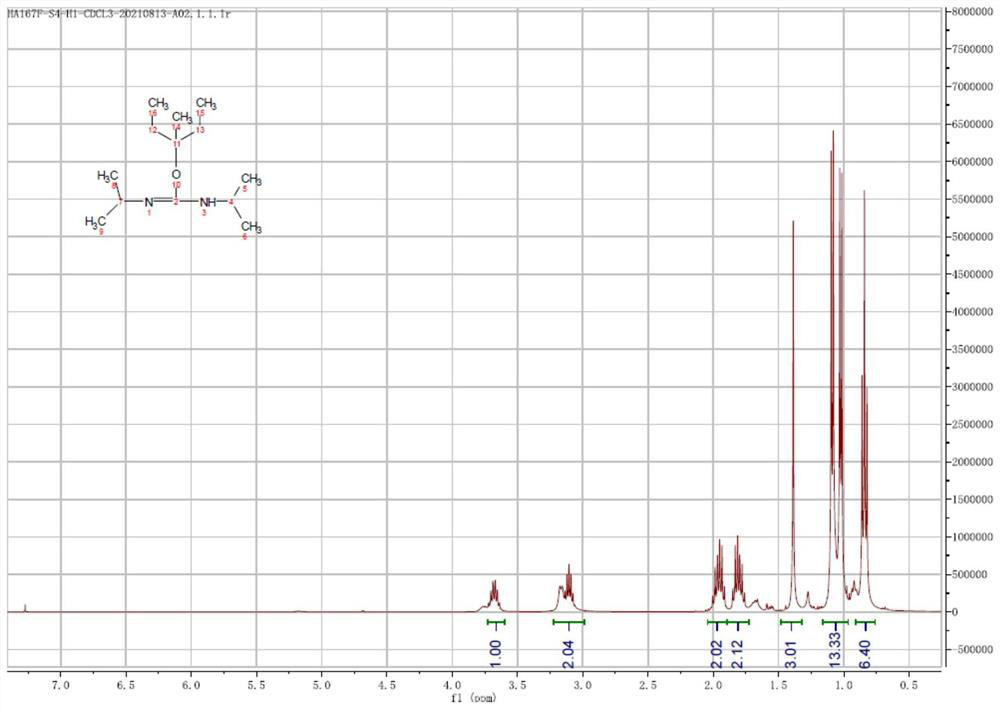
[0108] The structure of the multi-active compound is tested and analyzed by NMR, and the NMR spectrum is as follows figure 1 As shown, the structural formula of the compound can be obtained from the NMR spectrum as:
[0109]
Embodiment 2
[0111] A preparation method of an amino acid protected by a polyalkyl-substituted-3-pentanol group, the preparation steps comprising:
[0112] S1: At 25°C, in a 10L reaction flask equipped with a thermometer, sequentially add 3L tetrahydrofuran, 3L water, 300g sodium bicarbonate, 350g aspartic acid-1-benzyl ester, 580g FmocOSu, and stir for 14h at 25°C. The liquid was adjusted to weakly acidic with an aqueous hydrochloric acid solution with a molar concentration of 3 mol / L, 1 L of ethyl acetate was added to extract the aqueous phase twice, the organic phases were combined, washed once with saturated sodium chloride, dried over anhydrous sodium sulfate, concentrated, and washed with acetic acid. Ethyl ester petroleum ether was crystallized to obtain 320g Fmoc-aspartic acid-1-benzyl ester;
[0113] S2: At 25°C, in a 10L reaction flask equipped with a thermometer, add 3.2L tetrahydrofuran, 320g Fmoc-aspartic acid-1-benzyl ester, dropwise add 4.5kg of the active compound in Exampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com