Self-immobilized micromolecular fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and small molecules, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and luminescent materials, etc. It can solve the problems that the fluorescent signal cannot be retained and in-situ detection cannot be realized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, the synthesis of compound (1-1)
[0032]
[0033] 1. Synthesis of compound (2-3)
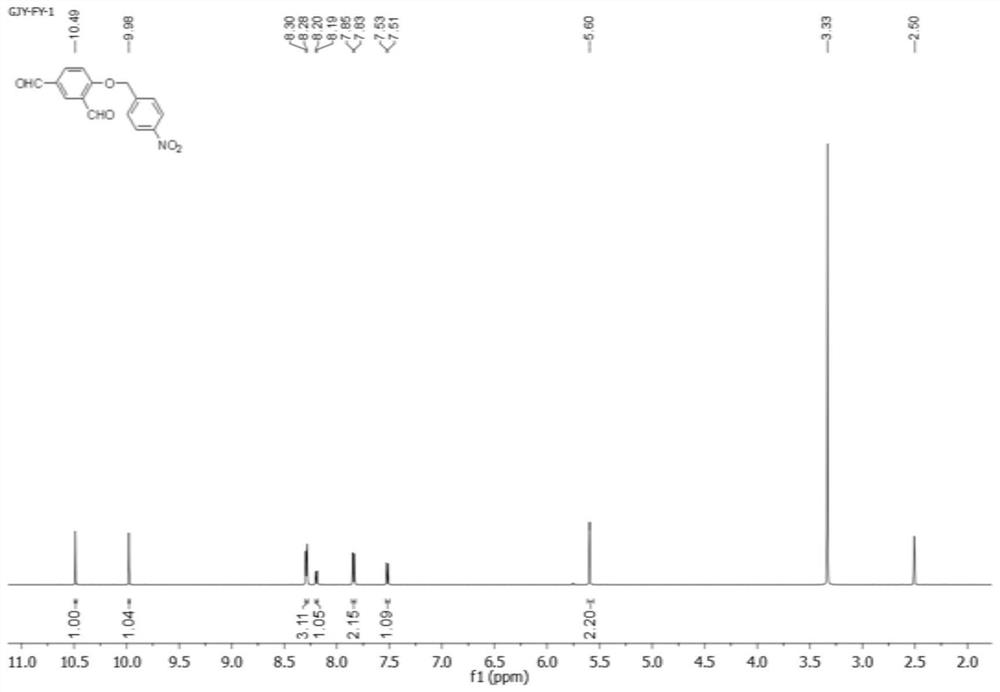
[0034] 4-Hydroxyisophthalaldehyde (2-1, 426 mg, 2.8 mmol) and p-nitrobenzyl bromide (2-2, 500 mg, 2.34 mmol) were dissolved in 10 mL of N,N-dimethylformamide (DMF ), followed by adding 415mg of potassium carbonate, stirring and mixing, adding 50mL of water and filtering (the filter membrane adopts ordinary qualitative filter paper), and drying the filter cake at 50°C for 24h to obtain 533mg of the product, namely the compound shown in formula (2-3). Rate 80%.
[0035] H NMR spectrum see figure 1 shown.
[0036] 1 HNMR(600MHz,DMSO)δ10.49(s,1H),9.98(s,1H),8.28-8.30(m,3H),8.19(d,J=8.7Hz,1H),7.84(d,J=8.6 Hz,2H),7.52(d,J=8.7Hz,1H),5.60(s,2H).
[0037] 2. Synthesis of compound (2-4)
[0038]
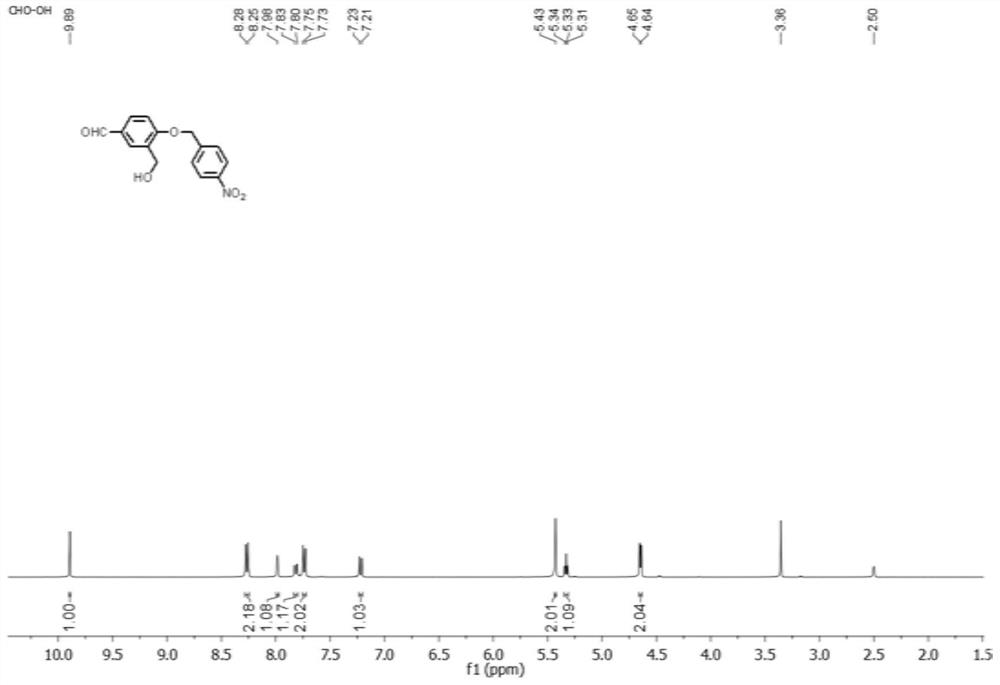
[0039] At room temperature, the compound represented by formula (2-3) (50 mg, 0.17 mmol) was dissolved in a mixed solvent (dichloromethane / methanol=8:1, v / v, 10 mL), and placed ...
Embodiment 2
[0046] Embodiment 2, the synthesis of compound (I)
[0047]
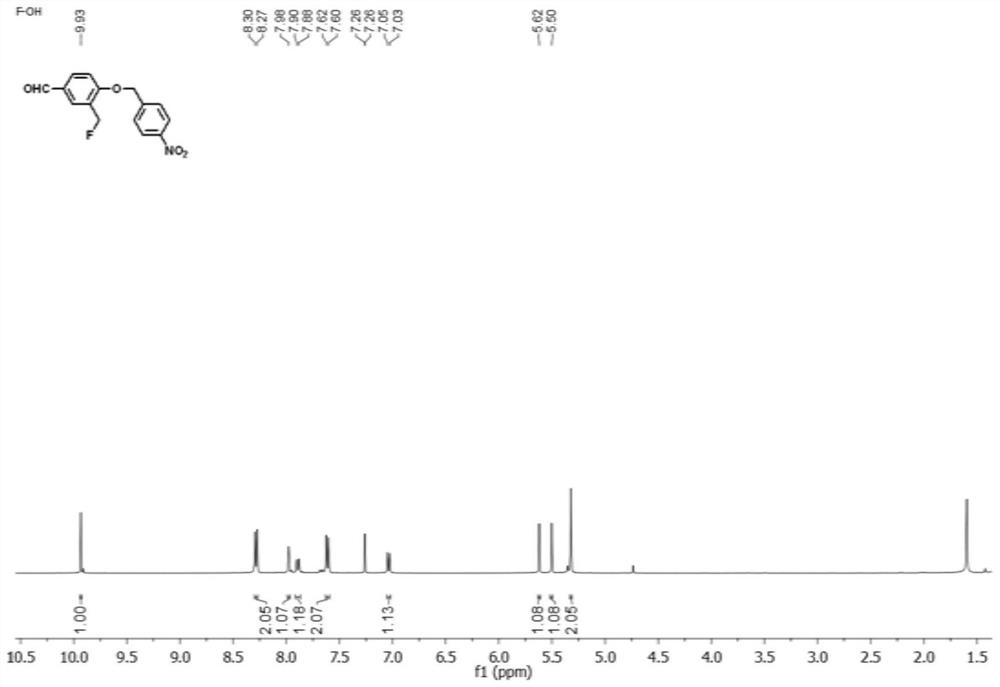
[0048] 1-Ethyl-2,3,3-trimethylindolium iodide (1-2, 9mg, 0.05mmol) and the compound shown in formula (1-1) prepared in Example 1 (15mg, 0.05mmol ) was dissolved in 4mL of ethanol, then sodium acetate (38mg, 0.46mmol) was added, reacted at room temperature for 4 hours, and the reactant was carried out through a silica gel chromatography column (column height 35cm, diameter 305nm, column height 10cm, silica gel particle size 300 mesh) Separation, using dichloromethane / methanol=80:1 (v / v) as the eluent, the elution rate is 0.3mL / s, eluting 5 column volumes, using dichloromethane / methanol with a volume ratio of 30:1 Thin-layer chromatography monitoring was carried out as a developing agent, and the effluents with Rf of 0.2-0.3 were combined, evaporated under reduced pressure to remove the eluent, and then dried in vacuo at 40°C to obtain 5 mg of the product, which was the small molecule probe shown in formula (I). F...
Embodiment 3
[0051] Example 3, Evaluation of the specific labeling effect of probe FY on Escherichia coli nitroreductase
[0052] 1. Nitroreductase (NTR)
[0053] References (JingBai, Altering the Regioselectivity of a Nitroreductase in the Synthesis of Arylhydroxylamines by Structure-Based Engineering, ChemBioChem, 2015, 16, 1219-1225.), using a nitroreductase-containing Escherichia coli strain to obtain liquid nitroreductase , the protein content is 1.8mg / mL, the specific expression and purification process of nitroreductase is as follows:
[0054] (1) Cultivation of nitroreductase strain
[0055] LB liquid medium formula (take 1L as an example): peptone 10g, yeast extract 5g, sodium chloride 5g, add ultrapure water to 1L.
[0056] LB solid medium formula (taking 1L as an example): peptone 10g, yeast extract 5g, sodium chloride 5g, agar powder 15g, ultrapure water to 1L.
[0057] Streak the plate to obtain a single colony: add Kana (kanamycin) to the sterilized LB solid medium at a vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com