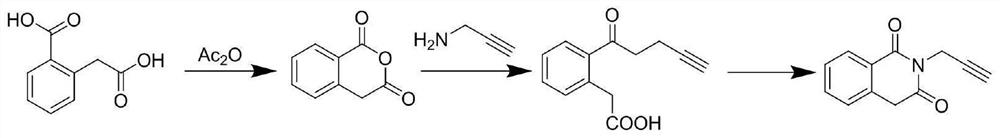
Preparation method of N-propyl-2-alkynyl phthalimide
A technology of alkynyl phthalimide and alkynyl benzamide, which is applied in the field of preparation of N-propyl-2-alkynyl phthalimide, can solve the problem of low market supply, low supply Low yield, unsuitable for large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of preparation method of N-propyl-2-alkynyl phthalimide, comprising the following steps:
[0024] (1) 2-formic acid phenylacetic acid is mixed with acetic anhydride, and dehydration reaction is carried out to obtain phthalic anhydride;
[0025] (2) The phthalic anhydride, propargylamine and phthalic anhydride good solvent are mixed, and ammonolysis reaction is carried out to obtain N-propyl-2-alkynyl benzamide-2-acetic acid;
[0026] (3) The N-propyl-2-alkynyl benzamide-2-acetic acid is mixed with a good solvent of N-propyl-2-alkynyl benzamide-2-acetic acid, and a ring-forming reaction is carried out to obtain N -Propyl-2-ynylphthalimide.
[0027] In the present invention, unless otherwise specified, the raw materials used are commercially available products well known to those skilled in the art.
[0028] The present invention mixes 2-formic acid phenylacetic acid and acetic anhydride for dehydration reaction to obtain phthalic anhydride...
Embodiment 1
[0037] (1) Add 1mol 2-formic acid phenylacetic acid and 25mol acetic anhydride to the reactor and stir evenly, then raise the temperature of the reactor to 80°C for reflux reaction for 3 hours, and monitor the reaction progress by TLC; after the reaction, cool the reactor to room temperature , and then the reaction solution was rotary evaporated at 35° C. to remove acetic anhydride to obtain a crude product, and then washed the crude product solid with 10 mL of petroleum ether to remove residual acetic anhydride on the surface to obtain phthalic anhydride. The yield was 88.90%, and the purity was 99%.
[0038] (2) Add 5g of phthalic anhydride and 25mL of tetrahydrofuran into the reactor and stir evenly, then add 2g of propargylamine dropwise to the solution at a rate of 10mL / min, after the addition is complete, react at 30°C for 12h, TLC The progress of the reaction was monitored; after the reaction was completed, the reaction solution was rotary evaporated at 35° C. to remove...
Embodiment 2
[0041] (1) Add 1mol 2-formic acid phenylacetic acid and 20mol acetic anhydride to the reactor and stir evenly, then raise the temperature of the reactor to 75°C for reflux reaction for 4 hours, and monitor the reaction progress by TLC; after the reaction, cool the reactor to room temperature , and then the reaction solution was rotary evaporated at 38° C. to remove acetic anhydride to obtain a crude product, and then the crude product solid was washed with 8 mL of petroleum ether to remove residual acetic anhydride on the surface to obtain phthalic anhydride. The yield was 87.60%, and the purity was 98.7%.
[0042] (2) Add 4g of phthalic anhydride and 20mL of methanol into the reactor and stir evenly, then add 1.7g of propargylamine dropwise to the solution at a rate of 12mL / min, after the addition is complete, react at 35°C for 13h, The progress of the reaction was monitored by TLC; after the reaction was completed, the reaction solution was rotary evaporated at 38° C. to rem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com