Organic luminescent material and preparation method thereof
A luminescent material and organic technology, applied in the directions of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of low quantum yield, and achieve the effects of high quantum dot yield, good heat resistance, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] In some embodiments of the present application, the preparation method of the compound of formula 2 comprises: providing the compound of formula 3 (phenothiazine) and the compound of formula 4, and obtaining the compound of formula 2 after heating and reacting;
[0054]
[0055] Wherein, R is selected from -H, -F, -CF 3 or -OCH 3 .
[0056] Further, the molar ratio of the compound of formula 3 to the compound of formula 4 may be (1-2):1, (1.1-1.9):1, or (1.2-1.8):1.
[0057] During specific implementation, the compound of formula 3 and the compound of formula 4, bis(dibenzylideneacetone) palladium (0), and tri-tert-butylphosphine tetrafluoroborate are dissolved in 1,4-dioxane solvent, Heat the reaction at 100°C for 7-12 hours to obtain the compound of formula 2.
[0058] In addition, the embodiment of the present application also provides an application of the above-mentioned organic light-emitting material or the organic light-emitting material prepared by the ab...
Embodiment 1
[0061] The organic light-emitting material with high quantum yield provided in this example includes xanthone, and is adjuvanted with 10-(4-fluorophenyl)phenothiazine. The steps for preparing the organic light-emitting material by the solvent method in this example are as follows:
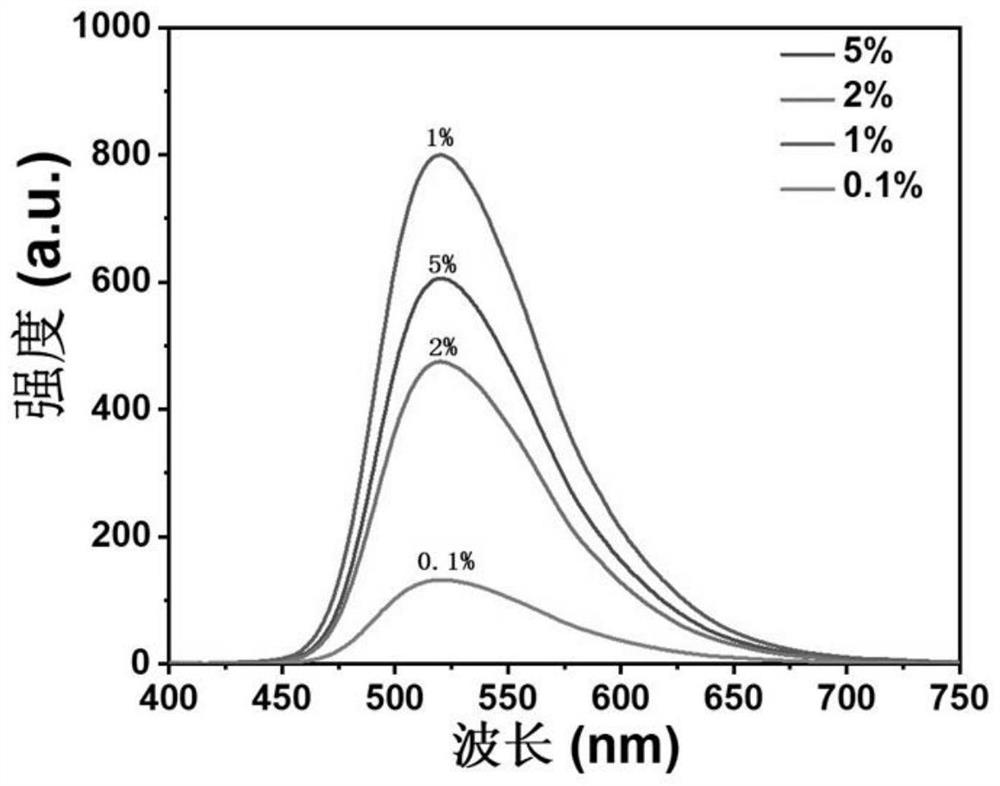
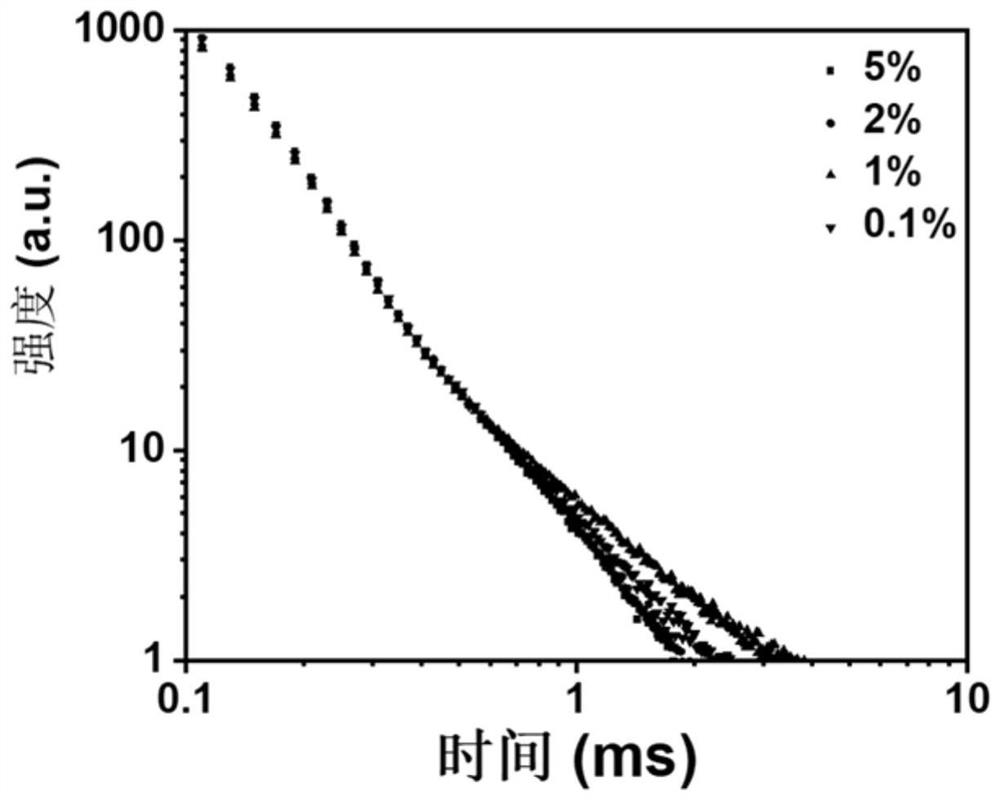
[0062] 1) Weigh 10-(4-fluorophenyl)phenothiazine and xanthone (purchased directly from the reagent company) respectively (1.6mg+1.071g, 3.96mg+265mg, 4.33mg+144.97mg, 10.26mg+137.4mg ), based on the total moles of xanthone and adjuvant, the molar percentage of 10-(4-fluorophenyl)phenothiazine is 0.1%, 1%, 2%, 5%, dissolved in dichloromethane solution;
[0063] 2) Spin the solution to obtain an organic luminescent material with green luminescence.
[0064] The preparation method of 10-(4-fluorophenyl) phenothiazine in step 1) is as follows:
[0065] (1) Phenothiazine (362mg, 1.82mmol), p-bromofluorobenzene (289mg, 1.65mmol), bis(dibenzylideneacetone) palladium (0) (48mg, 0.085mmol), tri-tert-butylp...
Embodiment 2
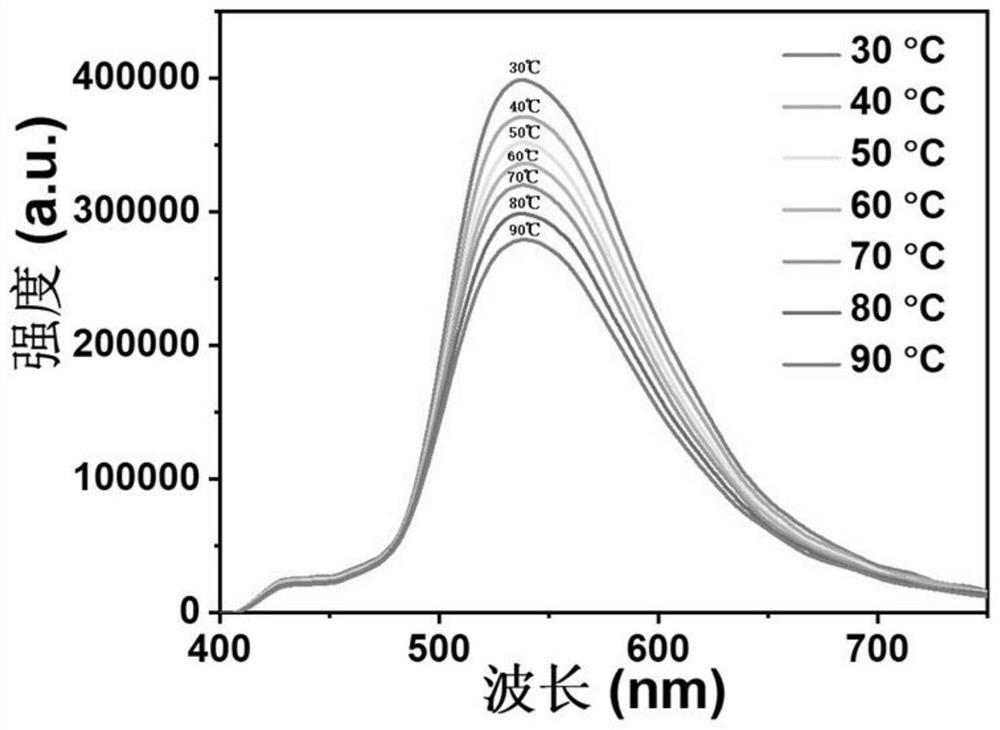
[0071] The light-emitting and high-temperature-resistant organic light-emitting material provided in this embodiment includes xanthone, and 10-(4-methoxyphenyl)phenothiazine is used as an adjuvant. In this embodiment, the steps of preparing organic luminescent materials by melting method are as follows:
[0072] 1) Weigh 3.28 mg 10-(4-methoxyphenyl) phenothiazine and 211 mg xanthone (purchased directly from a reagent company) in a sealed reaction bottle filled with argon;
[0073] 2) heating to 120° C. to melt and stir evenly, and cooling to room temperature to prepare an organic luminescent material.
[0074] The preparation method of 10-(4-methoxyphenyl) phenothiazine in step 1) is as follows:
[0075] (1) Phenothiazine (177mg, 0.89mmol), p-bromoanisole (166mg, 0.89mmol), bis(dibenzylideneacetone) palladium (0) (40mg, 0.067mmol), tri-tert-butylphosphine Tetrafluoroborate (20mg, 0.067mmol) was dissolved in 15ml of 1,4-dioxane solvent, and heated at 100°C for 12 hours.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com