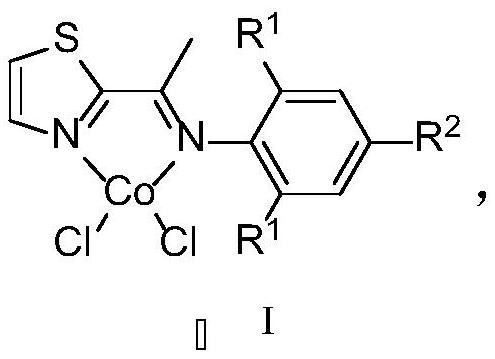
Thiazole imine-cobalt compound as well as synthesis method and application thereof
A technology of thiazole imines and cobalt compounds, which is applied in the field of catalysts for catalyzing the polymerization of isoprene and its synthesis, can solve problems such as unsatisfactory output, and achieve high activity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
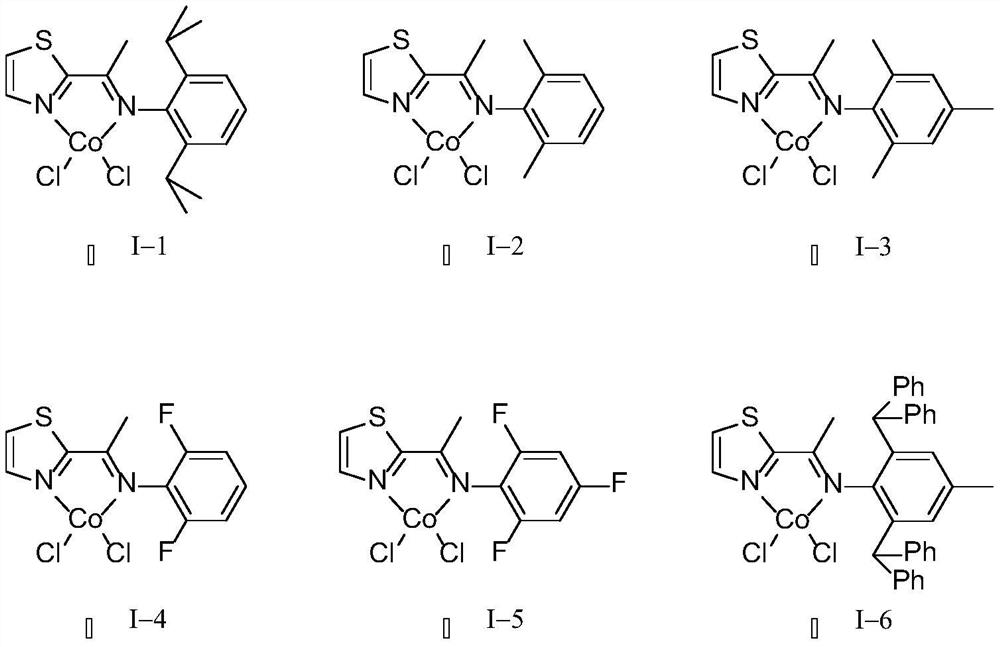
Embodiment 1
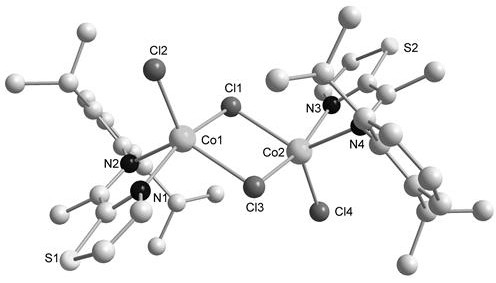
[0055] The synthetic method of present embodiment thiazole imine-cobalt compound Co1 is as follows:
[0056] (1) In a round bottom flask, add 2-acetylthiazole (2.54g, 20mmol), 2,6-diisopropylaniline (3.55g, 20mmol), 50mL methanol, then add a few drops of formic acid as a catalyst, and reflux 48h with continuous stirring; after the reaction, part of the solvent was removed, and then recrystallized at -30°C, filtered, and the obtained solid was washed with pre-cooled anhydrous methanol, and dried to obtain ligand L1. Yellow solid; Yield: 51%, 2.91g; 1 H NMR (600MHz, CDCl 3)δ7.95(d,J=3.2Hz,1H),7.49(d,J=3.2Hz,1H),7.18–7.13(m,2H),7.11(dd,J=8.5,6.7Hz,1H), 2.74(hept, J=6.8Hz, 2H), 2.23(s, 3H), 1.15(dd, J=6.9, 3.1Hz, 12H); 13 C{ 1 H}(151MHz, CDCl 3 )δ 170.25, 161.52, 144.98, 143.82, 136.03, 124.20, 123.19, 123.06, 28.45, 23.09, 22.82, 17.58.
[0057] (2) In a Shrek bottle, add L1 (285.6 mg, 1 mmol) and anhydrous cobalt chloride (129.8 mg, 1 mmol), then add 10 mL of tetrahydrofura...
Embodiment 2
[0060] Present embodiment thiazole imine-cobalt compound Co The synthetic method is as follows:
[0061] (1) The amount of 2-acetylthiazole in step (1) of Example 1 is replaced by (3.82g, 30mmol), and 2,6-diisopropylaniline is replaced by 2,6-methylaniline (3.64 g, 30mmol), other synthesis methods are the same as Example 1 step (1); the purification method is replaced by silica gel column chromatography, developing agent (petroleum ether / dichloromethane=100 / 1), and run column with triethylamine; Obtain Ligand L2. Yellow oily liquid; Yield: 4.21g, 61%; 1 H NMR (600MHz, CDCl 3 )δ7.94(d, J=3.2Hz, 1H), 7.49(d, J=3.2Hz, 1H), 7.06(d, J=7.5Hz, 2H), 6.95(t, J=7.5Hz, 1H) ,2.20(s,3H),2.05(s,6H); 13 C{ 1 H}(151MHz, CDCl 3 ) δ 170.05, 161.78, 147.26, 143.78, 127.91, 125.71, 123.65, 123.11, 17.91, 17.06.
[0062] (2) The synthesis of compound Co2 is the same as the step (2) of Example 1. Green solid; Yield: 234.3mg, 64%; Elemental analysis: C 13 h 14 Cl 2 CoN 2 S: C, 43.35; H, 3...
Embodiment 3
[0064] The synthetic method of present embodiment thiazole imine-cobalt compound Co is as follows:
[0065] (1) Replace the amount of 2-acetylthiazole in step (1) of Example 1 with (1.27g, 10mmol), and replace 2,6-diisopropylaniline with 2,4,6-trimethyl Aniline (1.35g, 10mmol), other synthetic methods are the same as Example 1 step (1); the purification method is replaced by silica gel column chromatography, developing agent (petroleum ether / methylene chloride=40 / 1), and moistened with triethylamine column; Ligand L3 was obtained. Orange-yellow solid; Yield: 1.56g, 64%; 1 H NMR (600MHz, CDCl 3 )δ7.95(d,J=3.2Hz,1H),7.54(d,J=3.2Hz,1H),7.11–7.04(m,1H),7.00–6.93(m,2H),2.42(s,3H ); 13 C{ 1 H}(151MHz, CDCl 3 )δ170.23, 161.94, 144.78, 143.73, 132.89, 128.57, 125.55, 123.00, 20.74, 17.84, 17.00.
[0066] (2) The synthesis of compound Co3 is the same as step (2) of Example 1. Dark green solid; Yield: 293.1g; 78%; Elemental analysis: C 14 h 16 Cl 2 CoN 2 S: C, 44.94; H, 4.31;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com