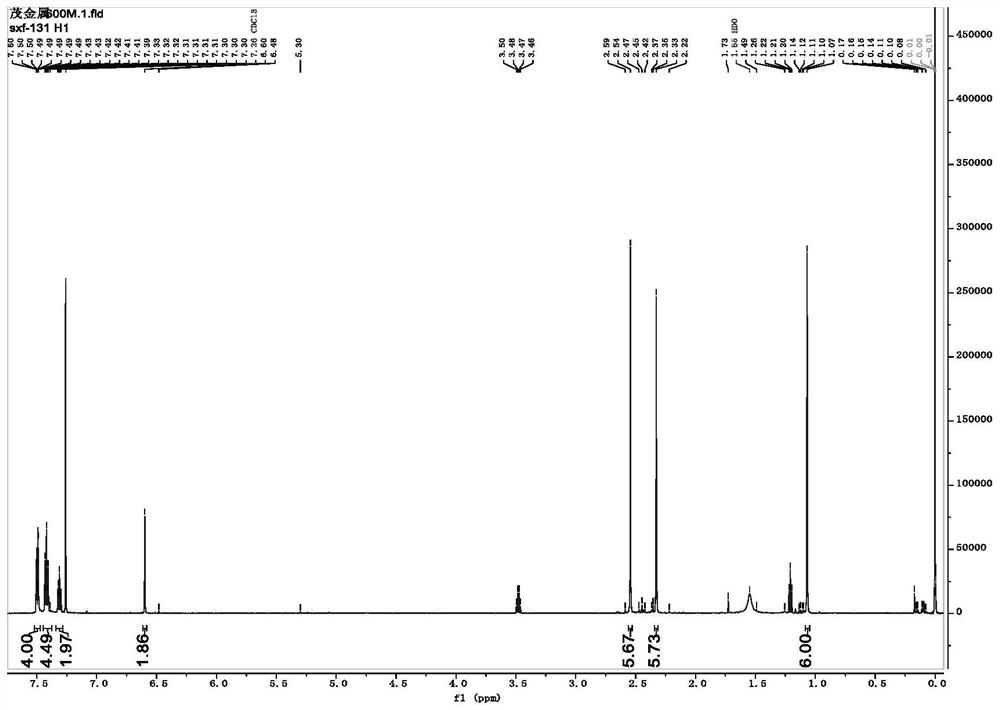
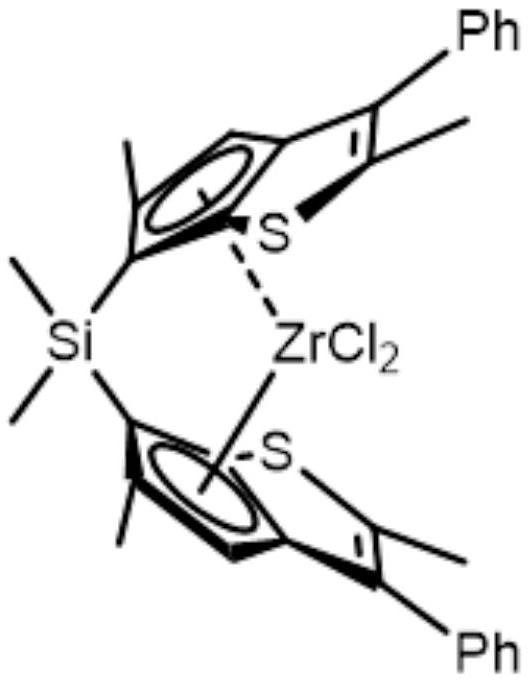
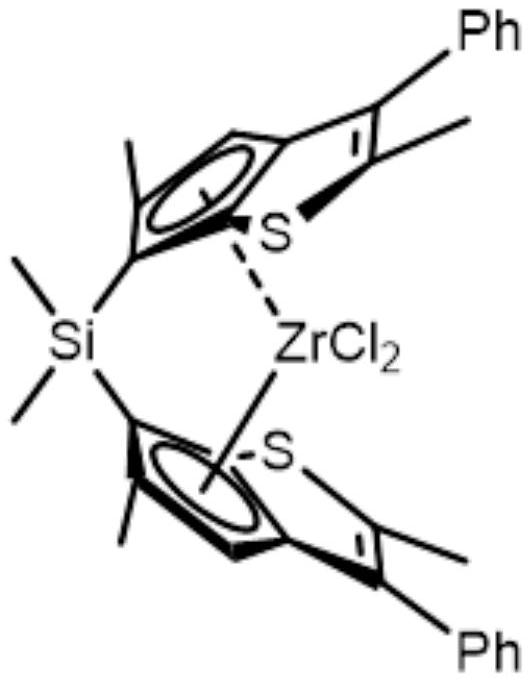
Synthesis method of zirconocene olefin polymerization catalyst
A technology for olefin polymerization and synthesis methods, which is applied in chemical instruments and methods, metallocenes, organic chemistry, etc. It can solve the problems of complex and harsh conditions, high cost, and high energy consumption, and achieve mild process conditions, low cost, and low energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1, the synthesis of 2-methyl-3-bromothiophene
[0031]
[0032]Dissolve diisopropylamine (56ml, 363mmol) in 200ml of anhydrous tetrahydrofuran, cool to -78°C, add n-butyllithium (2.5M n-hexane solution, 140ml, 330mmol), after the addition is complete, continue at -78°C Stir for 15min. To the solution containing lithium diisopropylamide (LDA) was added dropwise 3-bromothiophene (50 g, 307 mmol). After the addition was complete, the reactant was transferred to an ice bath, allowed to rise to 0°C naturally, and stirred for another 30 min. The reactant was cooled to -78°C, and iodomethane (21ml, 330mmol) was slowly added dropwise. The reaction mixture was stirred at -78 °C for another 30 min, then warmed to 0 °C and stirred for 1 h. After the reaction was completed, it was quenched with saturated brine, separated, and the aqueous phase was extracted with dichloromethane, washed with water, separated, dried over magnesium sulfate, filtered, and the solvent was rem...
Embodiment 2
[0046] Step 1, the synthesis of 2-methyl-3-bromothiophene
[0047] Dissolve 3-bromothiophene (50g, 307mmol) in 200ml of anhydrous tetrahydrofuran, cool to -78°C, add lithium hexamethyldisilazide (2M tetrahydrofuran solution, 165ml, 330mmol), after the addition is complete, - Stirring was continued for 15 min at 78 °C. Then the reactant was transferred into an ice-water bath, allowed to rise to 0°C naturally, and stirred for another 30 min. The reactant was cooled to -78°C, and iodomethane (21ml, 330mmol) was slowly added dropwise. The reaction mixture was stirred at -78 °C for another 30 min, then warmed to 0 °C and stirred for 1 h. After the reaction was completed, it was quenched with saturated brine, separated, and the aqueous phase was extracted with dichloromethane, washed with water, separated, dried over magnesium sulfate, filtered, and the solvent was removed in vacuo. The residue was distilled under reduced pressure to obtain a colorless oily liquid (38.11 g, 70%)....
Embodiment 3
[0058] Step 1, the synthesis of 2-methyl-3-bromothiophene
[0059] Dissolve 3-bromothiophene (50g, 307mmol) in 200ml of anhydrous tetrahydrofuran, cool to -78°C, add sodium hexamethyldisilazide (2M tetrahydrofuran solution, 165ml, 330mmol), after the addition is complete, - Stirring was continued for 15 min at 78 °C. Then the reactant was transferred into an ice-water bath, allowed to rise to 0°C naturally, and stirred for another 30 min. The reactant was cooled to -78°C, and iodomethane (21ml, 330mmol) was slowly added dropwise. The reaction mixture was stirred at -78 °C for another 30 min, then warmed to 0 °C and stirred for 1 h. After the reaction was completed, it was quenched with saturated brine, separated, and the aqueous phase was extracted with dichloromethane, washed with water, separated, dried over magnesium sulfate, filtered, and the solvent was removed in vacuo. The residue was distilled under reduced pressure to obtain a colorless oily liquid (39.70 g, 73%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com