Synthesis method of sclareleaf sclareleaf diol compound
A technology of sclareol and a synthesis method, which is applied in the field of iridium-catalyzed hydrogenation synthesis of sclarediols, can solve the problems of low atom economy, troublesome post-processing, high price and the like, and achieves less catalyst consumption , reduce the reaction cost, the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The structural formula of L-1 is as follows:
[0047]
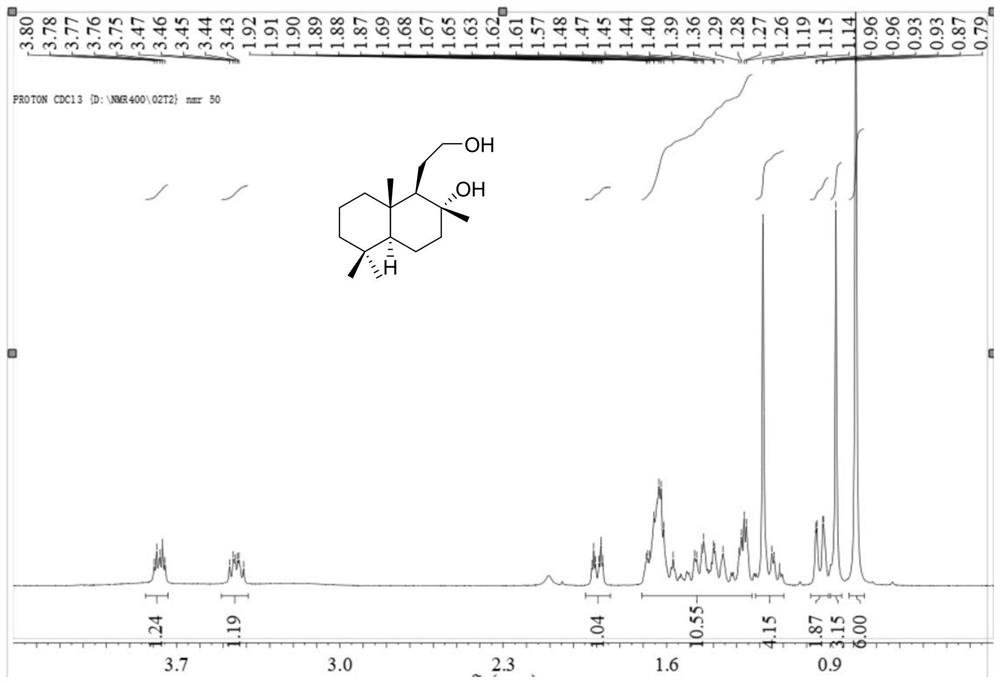
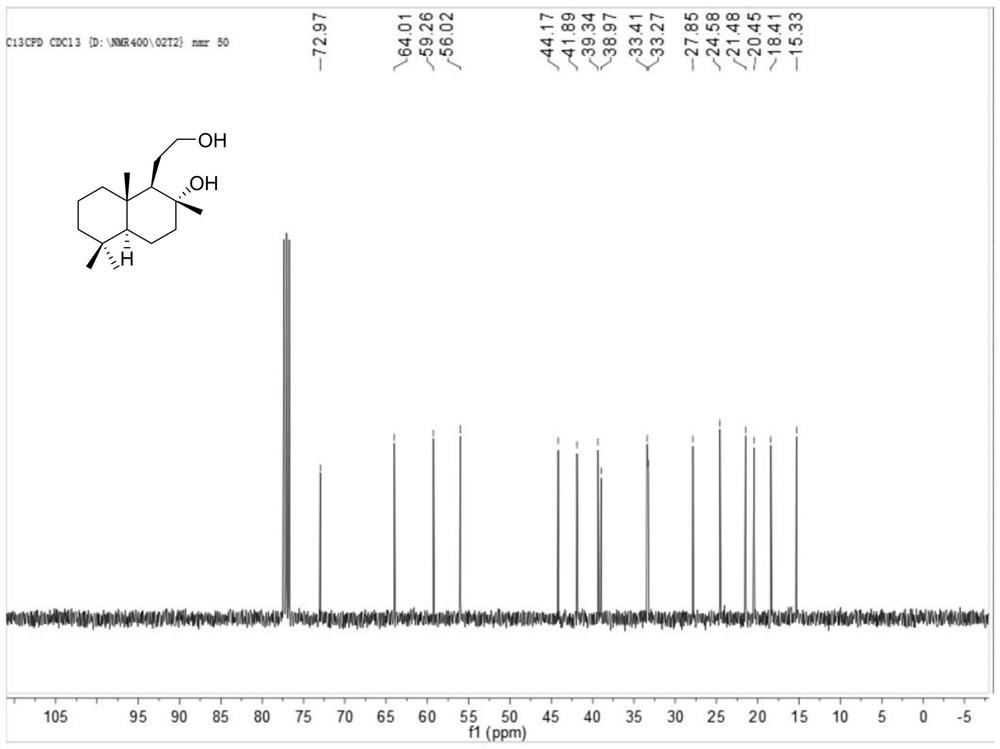
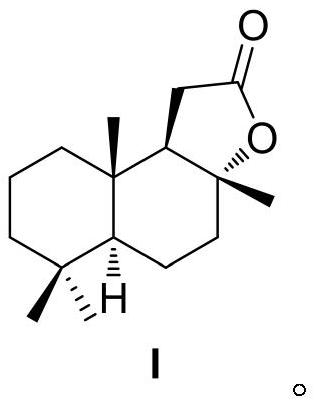
[0048] In the glove box, 1.3 mg [Ir(COD)Cl] 2 Dissolve 2.1mg of ligand L-1 in 2mL of methanol, stir at room temperature for 30 minutes, add it to 10g of sclareolactone, add 44.8mg of potassium tert-butoxide, 30mL of methanol, and place it in an autoclave In the process, the hydrogen gas was replaced 3 times, and then the hydrogen gas was introduced to 20 bar, and the reaction was carried out at 25°C for 12 hours, and the hydrogen gas was slowly released. After the solvent was removed, water was added, and then extracted with ethyl acetate, dried over sodium sulfate, and a white solid was obtained after the solvent was removed. Column chromatography, and then determined by NMR, obtained 4.3 g of sclareolactone and 5.2 g of sclareolide. Then the analytical method was established by GC analysis. The NMR data are as follows:
[0049] 1 H NMR (400MHz, CDCl 3 ( m,6H). 13 C NMR (400MHz, CDCl 3 )δ73.0, 64.0, 59.3,...
Embodiment 2
[0051] The structural formula of L-2 is as follows:
[0052]
[0053] The ligand L-1 in Example 1 is replaced by the ligand L-2 and the rest is the same as in Example 1. GC monitoring conversion was 70%.
Embodiment 3
[0055] The alkali additive potassium tert-butyl in embodiment 1 is replaced with lithium tert-butoxide, and all the other are the same as embodiment 1. GC monitoring conversion was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com