Protein-inorganic nanoflower with controllable protein distribution and size as well as preparation method and application of protein-inorganic nanoflower
A protein distribution and inorganic nanotechnology, applied in the field of biosensors, can solve problems such as poor repeatability of analysis results, achieve effective immobilization, good biocompatibility, and improve sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: A preparation method of protein-inorganic nanoflowers with controllable protein distribution and size, comprising the following steps:
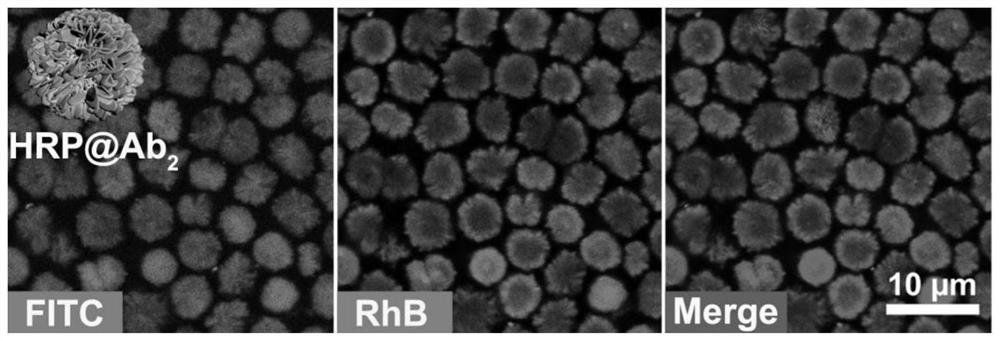
[0030] (1) FITC-labeled HRP (FITC-HRP) and RhB-labeled Ab 2 (RhB-Ab 2 ) preparation method refer to the literature Chem.Mater.2018, 30, 1069-1077. Next, use FITC-HRP and RhB-Ab 2 Protein-inorganic nanoflowers were synthesized. First, 30 μL, 200 mmoL -1 The copper sulfate aqueous solution was added to the centrifuge tube, to which was added 1 mL containing 1.0 mg mL - 1 Phosphate buffer solution of FITC-HRP (0.01 mmol -1 , pH 7.4). After vortexing for 5 minutes, it was left to react at 4°C for 12 hours.
[0031] (2) After taking out, at 1000r min -1 Centrifuge for 1 min, dilute to 1 mL with deionized water, and set it at 1000 rpm. -1 Centrifuge again for 1 min at rpm and remove the supernatant. Then, 1 mL containing 0.25 mg mL was added thereto -1 RhB-Ab 2 phosphate buffered solution (0.01 mmol -1 , pH 7.0). ...
Embodiment 2
[0032] Embodiment 2: A preparation method of protein-inorganic nanoflowers with controllable protein distribution and size, comprising the following steps:
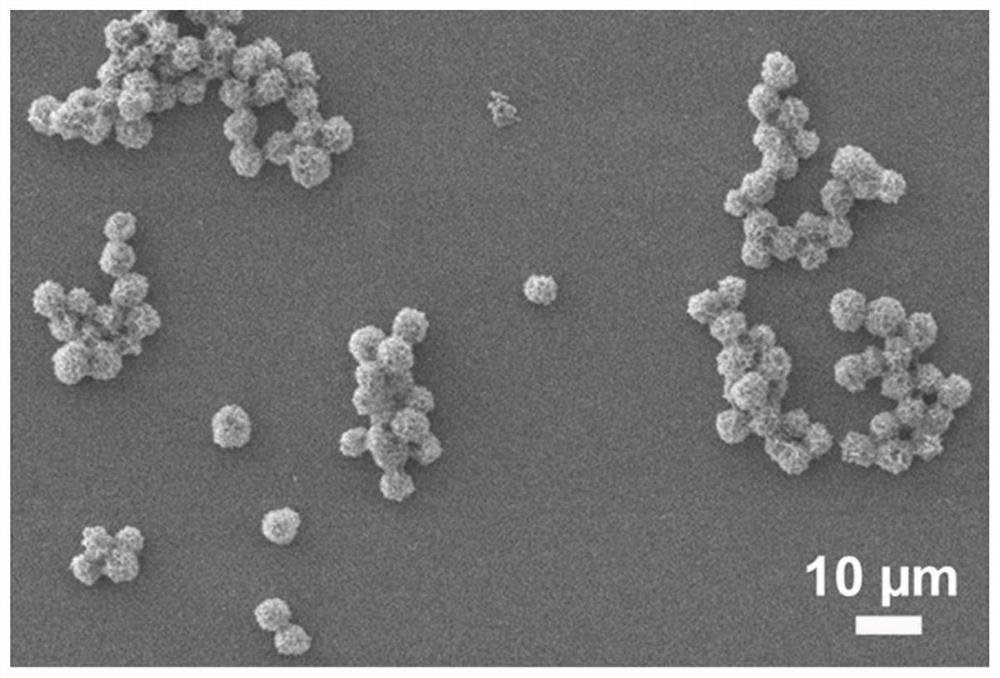
[0033] (1) First, 30 μL, 200 mmol -1 The copper sulfate aqueous solution was added to the centrifuge tube, and 1 mL containing 0.1 mg mL was added to it. -1 Phosphate buffer solution of HRP (0.01 mmol -1 , pH 7.4). After vortexing for 5 minutes, it was left to react at 4°C for 12 hours.
[0034] (2) After taking out, at 1000r min -1 Centrifuge for 1 min, dilute to 1 mL with deionized water, and set it at 1000 rpm. -1 Centrifuge again for 1 min at rpm and remove the supernatant. Then, 1 mL containing 0.25 mg mL was added thereto -1 Ab 2 phosphate buffered solution (0.01 mmol -1 , pH 7.0). After 5 minutes of vortexing, it was left to react at 4°C for 24 hours. Then at 1000r min -1 Centrifuge twice at a rotating speed for 2 minutes each time; then dilute to 1 mL with deionized water to obtain a protein-inorganic n...
Embodiment 3
[0035] Embodiment 3: A preparation method of protein-inorganic nanoflowers with controllable protein distribution and size, comprising the following steps:
[0036] (1) First, 30 μL, 200 mmol -1 The copper sulfate aqueous solution was added to the centrifuge tube, to which was added 1 mL containing 1.0 mg mL -1 Phosphate buffer solution of HRP (0.01 mmol -1 , pH 7.4). After vortexing for 5 minutes, it was left to react at 4°C for 12 hours.
[0037] (2) After taking out, at 1000r min -1 Centrifuge for 1 min, dilute to 1 mL with deionized water, and set it at 1000 rpm. -1 Centrifuge again for 1 min at rpm and remove the supernatant. Then, 1 mL containing 0.25 mg mL was added thereto -1 Ab 2 phosphate buffered solution (0.01 mmol -1 , pH 7.0). After 5 minutes of vortexing, it was left to react at 4°C for 24 hours. Then at 1000r min -1 Centrifuge twice at a rotating speed for 2 minutes each time; then dilute to 1 mL with deionized water to obtain a protein-inorganic nan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com