Synthesis method of Euphorbiaceae diterpene Peplanol A
A synthesis method and technology of Euphorbiaceae are applied in the field of synthesis of the diterpenoid PepluanolA of Euphorbiaceae, can solve the problems of few natural products and cannot be prepared in large quantities, and achieve the effects of easy availability of raw materials, novel design ideas and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
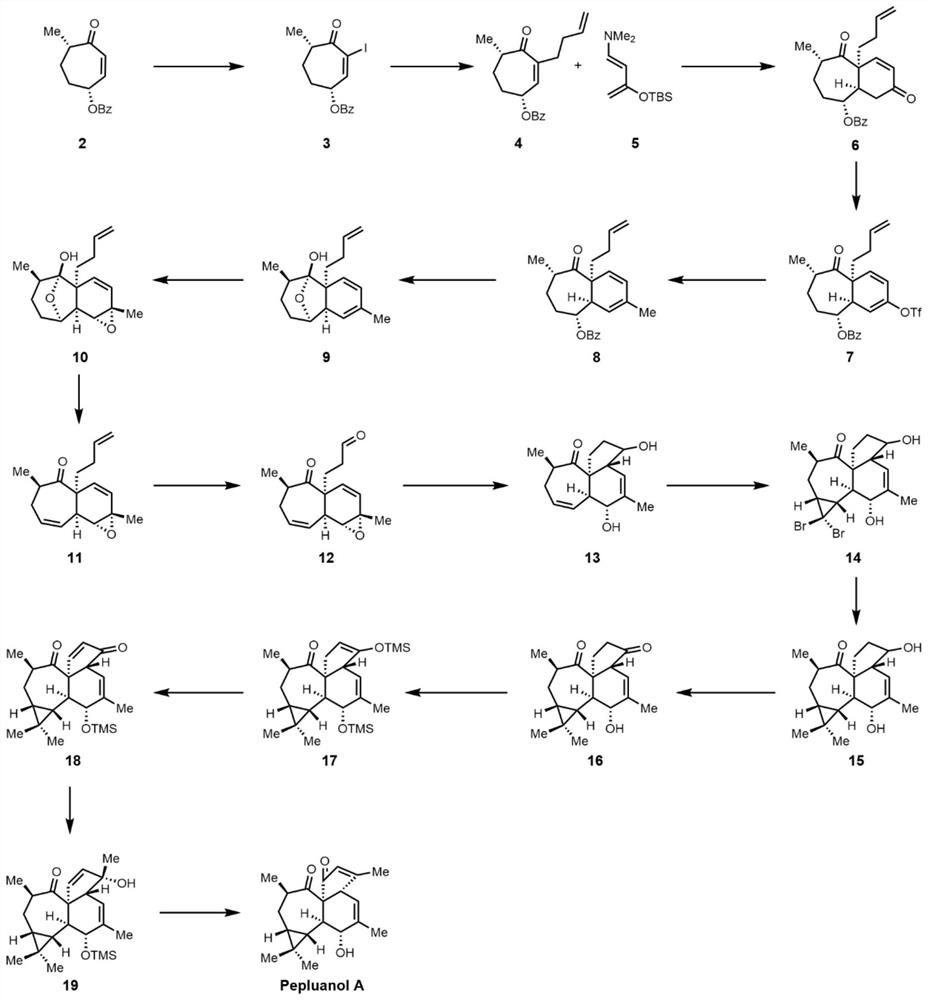
[0055] The synthetic route diagram of the Pepluanol A compound is shown in Figure 1.
[0057]
[0058] At 0 °C, to (20 g, 0.082 mol) of the dichloromethane (200 mL) solution of the enone compound 2 was added
[0060]
[0061]
[0065]
[0069]
[0070]
[0074]
[0077]
[0078]
[0082]
[0083] The diketone compound 6 shown in the above formula (11.94 g, 33.9 mmol) was added to a solution of tetrahydrofuran (200 mL).
[0085]
[0086] The trifluoromethanesulfonate compound 7 (15.6 g, 29.5 mmol) shown in the above formula was dissolved in tetrahydrofuran (200 mL).
[0088]
[0089]
[0093]
[0096]
[0099]
[0100]
[0104]
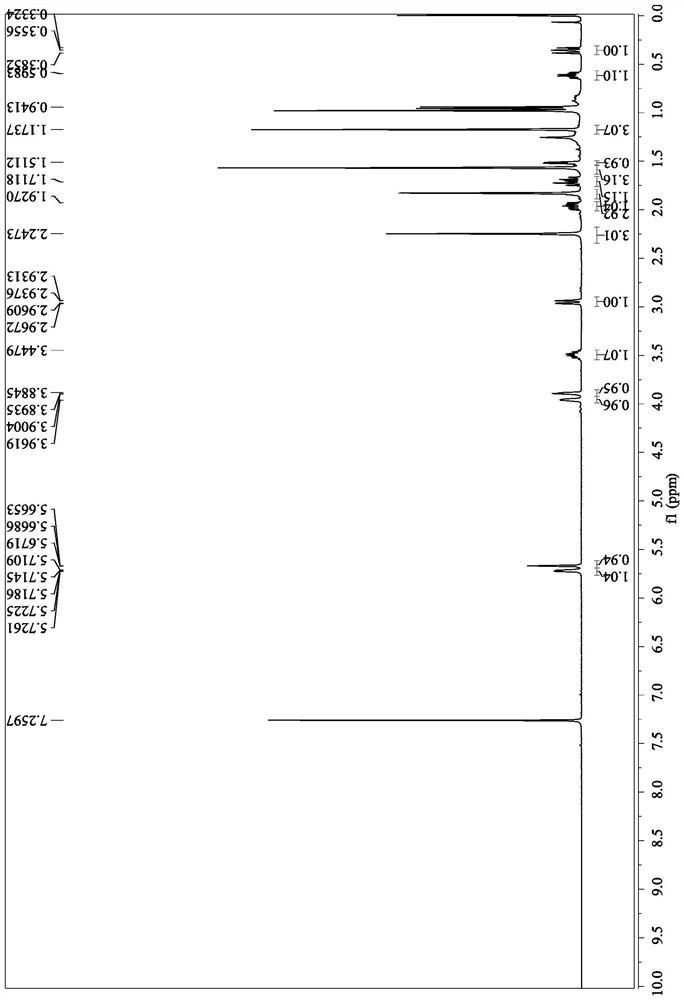
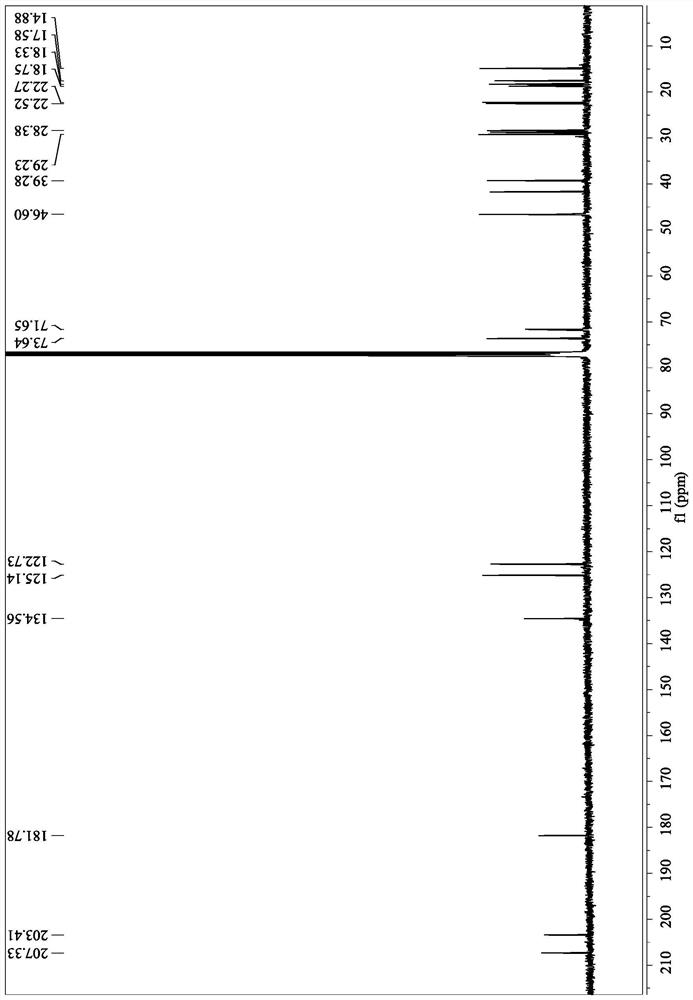
[0106] Nuclear magnetic data:
[0107]
[0108]
[0112]
[0114] Nuclear magnetic data:
[0115]
[0116]
[0120]
[0123]
[0124]
[0128]
[0131]
[0134]
[0135]
[0136] Diastereomer 1 high-resolution mass spectrometry data:
[0139]
[0140]
[0141] Diastereomer 2 high-resolution mass spectrometry data:
[0144]
[0146] Nuclear Magnetic Data:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com