Preparation method of milnacipran hydrochloride preparation
A technology of milnacipran hydrochloride and preparation, which is applied in the field of drug preparation to achieve the effects of shortening production cycle, ensuring stability and low production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
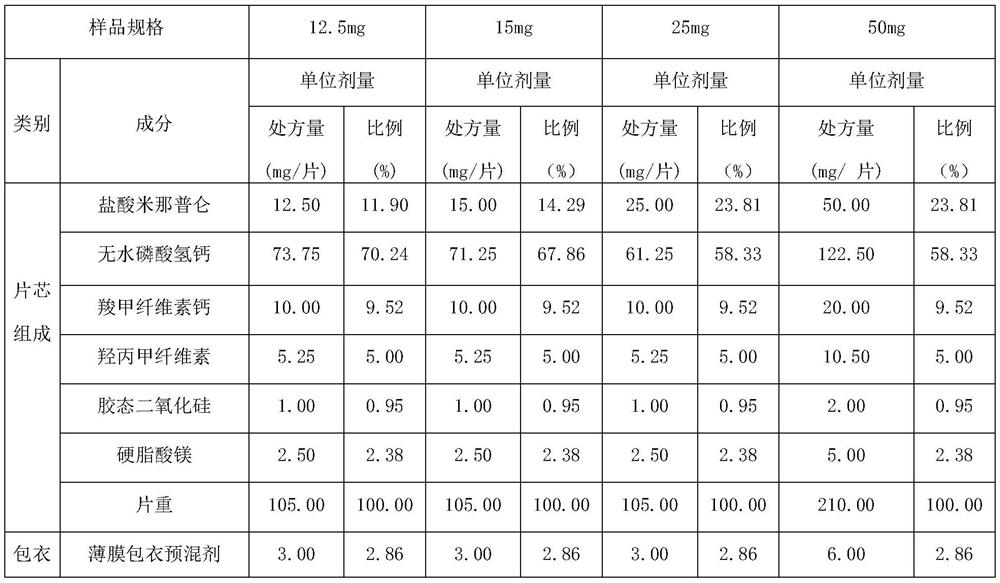
[0060] In this embodiment, the filler is anhydrous calcium hydrogen phosphate, the disintegrant is calcium carboxymethyl cellulose, the binder is hypromellose, the glidant is colloidal silicon dioxide, and the lubricant is stearic acid magnesium. According to the prescription composition shown in table 1, adopt the powder direct compression technology, respectively obtain the milnacipran hydrochloride specification (content of active ingredient in the unit preparation) that is 12.5mg, 15mg, 25mg, 50mg milnacipran hydrochloride tablets .
[0061] Table 1 Specifications 12.5mg, 15mg, 25mg, 50mg prescription composition
[0062]
[0063]
[0064] The specific preparation method is as follows:
[0065] (1) Pretreatment: The raw material drug (namely, milnacipran hydrochloride, with a particle size of 50-160 μm) is passed through a 0.45 mm sieve with a pulverizing and granulating machine for use.
[0066] (2) Weighing ingredients: Weigh the raw and auxiliary materials acco...
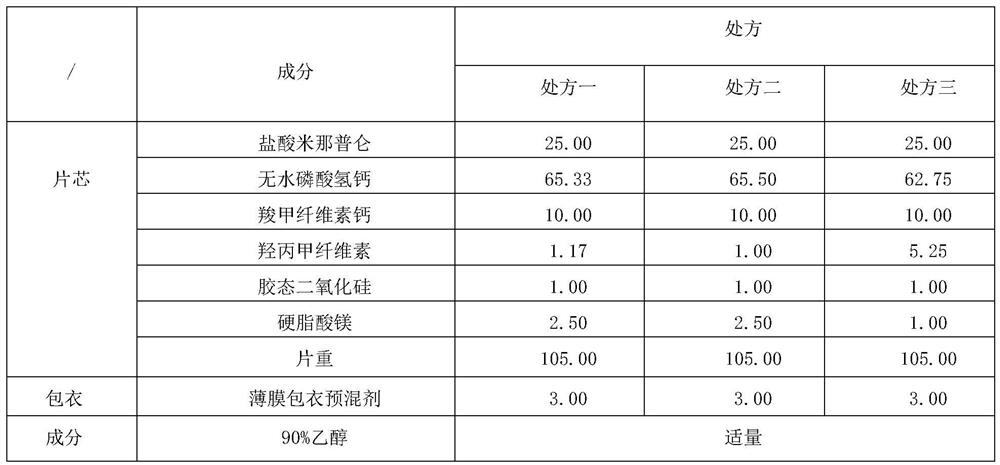
experiment example 1
[0093]Experimental Example 1: Influencing Factors Test of Milnacipran Hydrochloride Tablets
[0094] (1) Test method
[0095] The product stability of the milnacipran hydrochloride tablets of the present invention, the milnacipran hydrochloride tablets obtained by wet granulation, the commercial product and the reference preparation under the condition of high temperature of 60°C for 30 days was investigated.
[0096] sample:
[0097] Sample prepared in Example 1 (Milnacipran hydrochloride tablets obtained by powder direct compression, specifications: 12.5mg, 15mg, 25mg, 50mg);
[0098] The sample prepared by comparative example 1 (milnacipran hydrochloride tablets obtained by wet granulation process, specification: 25mg);
[0099] Commercial product (Milnacipran hydrochloride tablets, specification: 25mg, manufacturer: Shanghai Modern Pharmaceutical Co., Ltd.);
[0100] Reference preparation (Milnacipran hydrochloride tablets, specifications: 25 mg, 50 mg, manufacturer: As...
experiment example 2
[0111] Experimental Example 2: Accelerated Test of Milnacipran Hydrochloride Tablets
[0112] (1) Test method
[0113] Samples prepared in Example (Milnacipran hydrochloride tablets obtained by powder direct compression, specifications: 12.5mg, 15mg, 25mg, 50mg);
[0114] Samples prepared by comparative example (Milnacipran hydrochloride tablets obtained by wet granulation process, specification: 25mg); commercial product (Milnacipran hydrochloride tablets, specification: 25mg, manufacturer: Shanghai Modern Pharmaceutical Co., Ltd.); Specific formulation (Milnacipran hydrochloride tablets, specifications: 25 mg, 50 mg, manufacturer: Asahi Kasei Pharmaceutical Co., Ltd.).
[0115] experiment method:
[0116] The samples were sealed and packaged in polyethylene film plastic bags, placed in a constant temperature and humidity incubator at 40 °C ± 2 °C and a relative humidity of 75 ± 5%, placed for three months, and samples were taken at the end of 1, 2, and 3 months. And compa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com