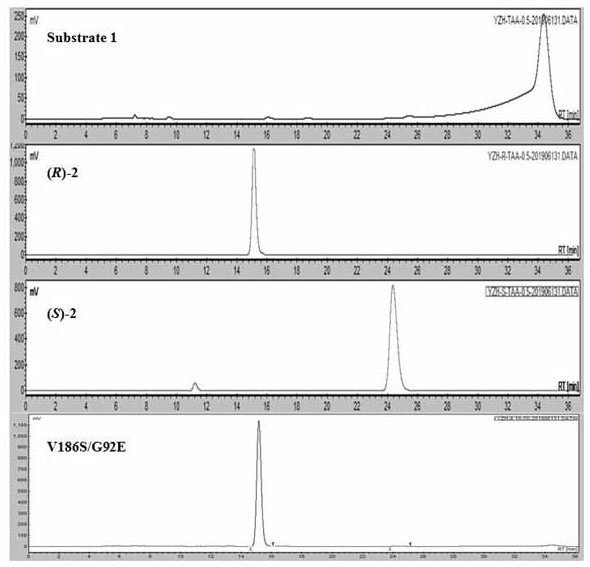
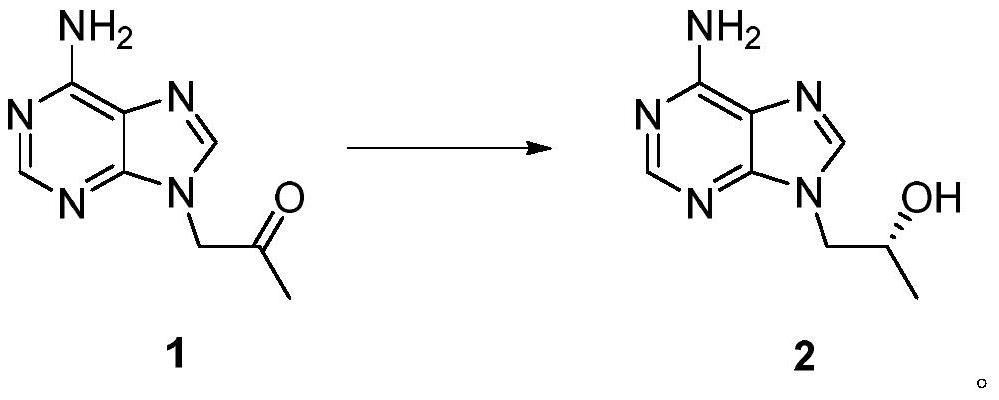
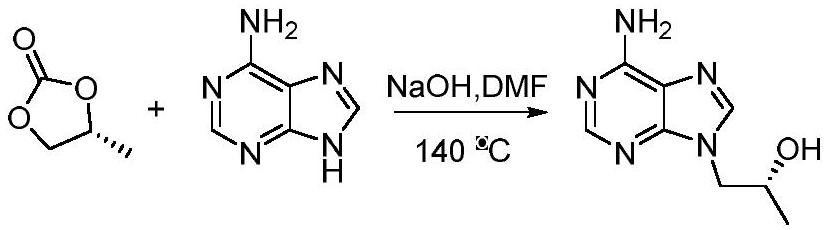
Short-chain dehydrogenase as well as mutant and application thereof
A short-chain dehydrogenase and mutant technology, applied in the field of medicine, can solve the problem of not using adenine, etc., and achieve the effects of high conversion rate, stereoselectivity, green environmental protection, and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Construction of mutants
[0054] Using the plasmid containing the short-chain dehydrogenase wild-type gene as a template, the mutant gene was obtained by overlapping extension PCR method.
[0055] The designed primers are as follows:
[0056]
[0057]
[0058] The PCR reaction system is as follows:
[0059]
[0060] Amplification program: 94°C: 10 min, (94°C: 30s, 48°C: 30s, 72°C: 90s) 35 cycles, 72°C, 10min.
Embodiment 2
[0061] Example 2 Construction of mutant gene recombinant plasmid
[0062] The mutant gene obtained by overlapping extension PCR was double digested with the vector plasmid pET22b, and the reaction system was as follows:
[0063]
[0064] After 4-8 hours of incubation in a 37°C incubator, use a common DNA product gel recovery kit to recover the digested nucleic acid fragments; use T4 DNA ligase to double-digest the mutated gene fragments and vectors with sticky ends The plasmid pET22b fragment was ligated (16°C for 2-6h) to obtain the recombinant plasmid.
Embodiment 3
[0065] Example 3 Preparation and transformation of competent cells E. coli Rosetta (DE3)
[0066] a) Take 1 mL of seed medium and inoculate it into 100 mL of LB liquid medium, and inoculate it with shaking at 37°C and 200 r / min for 3 hours;
[0067] b) 3000r / min, 10min to enrich the bacteria, discard the supernatant;
[0068] c) Add 400μL of pre-chilled 0.1M CaCl 2 solution, resuspend the bacterial cells, aliquot into 4 EP tubes, and take an ice bath for 30 min to obtain a competent cell suspension;
[0069] d) Add 20 μL of recombinant plasmid ligation solution to the competent cell suspension, mix gently, and take an ice bath for 30 minutes;
[0070] e) Heat shock at 42°C for 90s, ice-water bath for 3min, add 800μL of LB liquid medium, incubate at 37°C for 1-2h, shake at 200r / min for 1-2h;
[0071] f) Take 150 μL of culture solution and spread it on LB solid medium with kanamycin or ampicillin resistance respectively, and pick positive transformants after culturing at 37°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com