Protein purification
A protein and protein technology, applied in the field of protein purification, can solve problems such as unpredictability and different protein performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0115] The preparation and use of immunogenic compositions is well known to those of ordinary skill in the art. Liquid pharmaceutical compositions typically contain a liquid carrier such as water, petroleum, animal or vegetable oils, mineral oils or synthetic oils. Physiological saline solution, dextrose or other sugar solutions or glycols such as ethylene glycol, propylene glycol or polyethylene glycol may also be included.
[0116] Alternatively, vaccine injections can be prepared by stepwise freeze-drying of the virus in the formulation. In certain embodiments, the formulations comprise additional additives suitable for in vivo administration, such as mannitol, dextran, sugars, glycine, lactose, polyvinylpyrrolidone, or other additives, for example, including but not limited to antioxidants or inert Gases, stabilizers or recombinant proteins (eg human serum albumin). The ampoule is then sealed and can be stored at a suitable temperature (eg between 4°C and room temperatur...
example
[0126] The upstream and downstream processes of the production and purification of recombinant proteins expressed by the recombinant PER.C6 cell line (eg gp140 of clade C HIV (SEQ ID NO: 1) or mosaic HIV gp140 (SEQ ID NO: 2)) were investigated. Various growth media were tested to improve gene expression and productivity of recombinant host cells in bioreactors. For example, the feed to the bioreactor is concentrated by 20% to allow for increased production rates.
[0127] Various procedures, conditions and columns were investigated for purification of proteins of interest (e.g. gp140 of clade C HIV or mosaic HIV gp140) where the goal was, e.g., to minimize final host cell protein (HCP) levels, maintain product Variation levels and / or conformations, eliminate DNA and other contamination, and have relatively high yields (eg, at least about 10%, preferably at least about 15% overall yield). Using the methods of the present invention, HCP levels in the final gp140 protein product...
example 1
[0134] Example 1. Purification of clade C gp140
[0135] Clade C gp140 was produced by a fed-batch cell culture process. Expansion of cells and production of clade Cgp140 occurred during the first 2 stages of the process, including stage 1 (pre-culture and seed bioreactor) using the PER.C6 cell line expressing clade C gp140; and stage 2 (produced in bioreactors with volumes ranging from 15,000L to 16,500L). Subsequent purification and manufacture of the formulated stock solution (FB) takes place in the remaining 11 stages. image 3 A flow chart of the clade C gp140 drug substance manufacturing process from pre-culture and expansion to drug substance (DS) is shown.
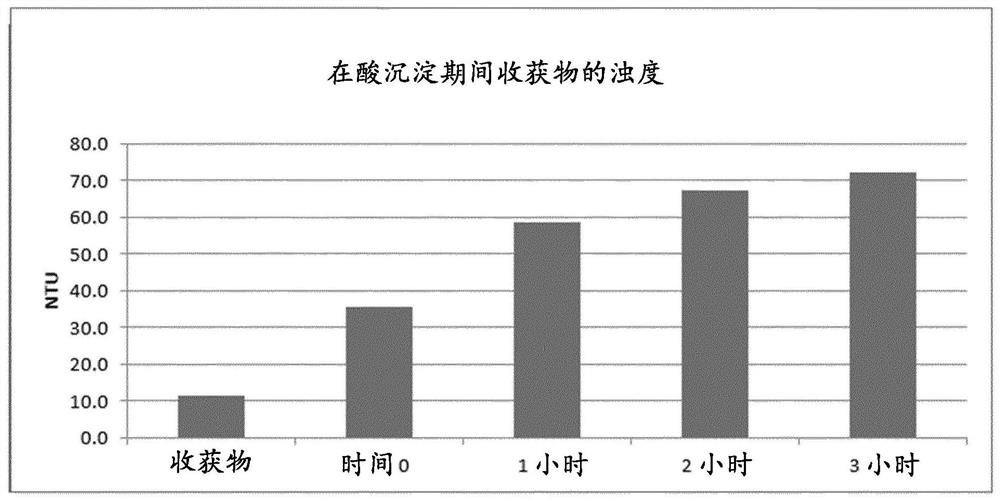
[0136] The target run duration for the 15,000L production process is 18 days. The 15,000 L produced contents were then flocculated by adjusting to a target pH of 4.8 with 25% acetic acid (stage 3, low pH flocculation). After flocculation, clarification by centrifugation and deep / finishing filtration (stage 4). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com