Triazole feed additive as well as preparation method and application thereof
A technology of feed additives and triazoles, which is applied in the field of feed additive synthesis to improve the body's immunity, reduce inflammation, and improve utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
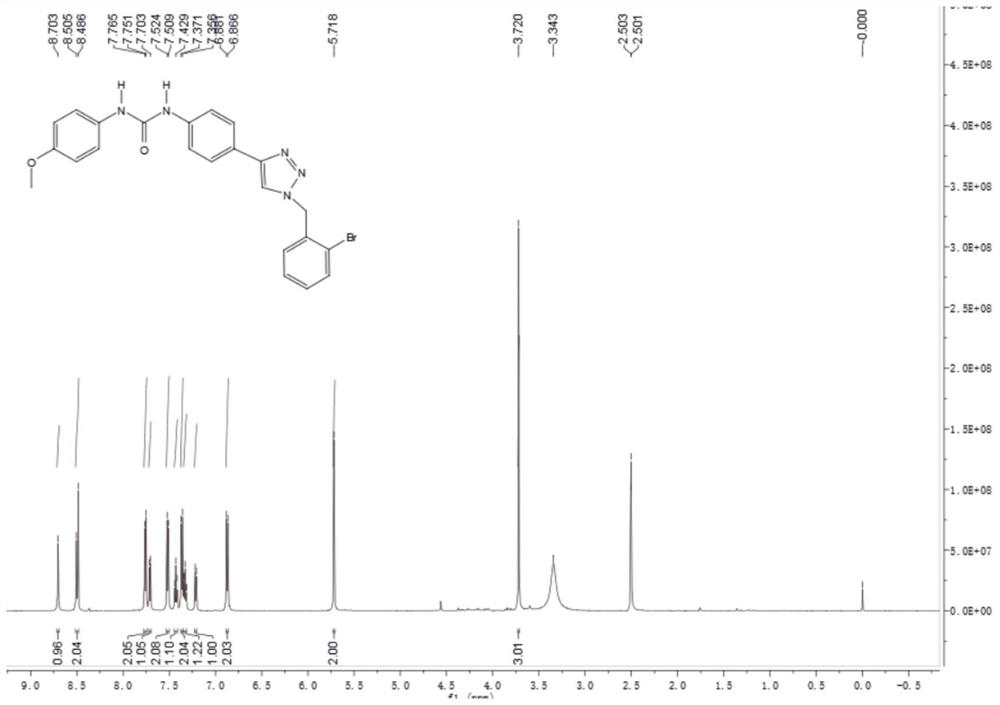
[0019]
[0020] In a reaction flask with nitrogen protection and temperature control device, 20 g of 3-methoxybenzyl bromide was added to 350 mL of acetonitrile, stirred evenly, and then cooled to 0 °C, and 250 mL of acetonitrile dissolved with 10 g of sodium azide was slowly added dropwise. After the dropwise addition, the mixture was stirred for 1 h, and then 1.9 g of cuprous iodide and 300 mL of a tert-butanol solution in which 11 g of trimethylsilylacetylene was dissolved were added to the reaction system, stirred to dissolve, and slowly heated to 50°C. 1000 mL of water was added, the reaction system was extracted with 200 mL of dichloromethane for several times, the organic phases were combined, dried over anhydrous sodium sulfate 50 g, concentrated, and separated by silica gel column chromatography to obtain 16.26 g of 3-methoxybenzyl triazole; LC-MS (ESI): m / z 190 [M+H] + .
Embodiment 2
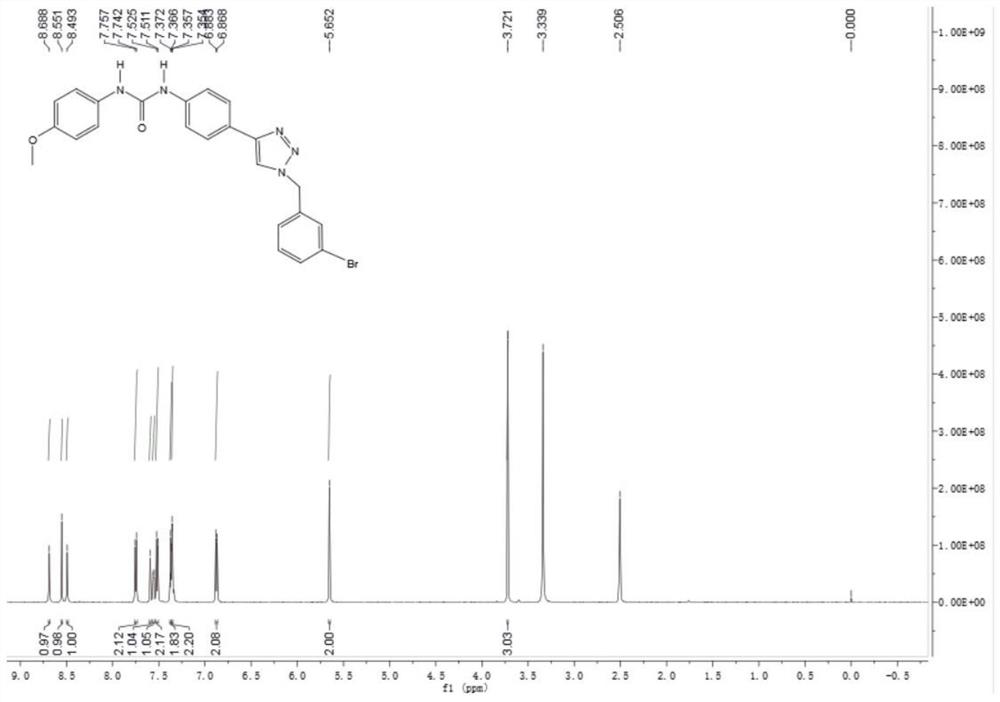
[0022]
[0023] In a reaction flask with nitrogen protection and temperature control device, add 25 g of 3-bromobenzyl bromide into 400 mL of acetonitrile, stir evenly, and then cool down to 0 °C, slowly add 250 mL of acetonitrile dissolved with 10 g of sodium azide dropwise, and add dropwise. After stirring for 1 h, add 1.9 g of cuprous iodide and 300 mL of tert-butanol solution dissolved in 11 g of trimethylsilyl acetylene into the reaction system, stir to dissolve, slowly heat to 50 ° C, and add water to the reaction system after the reaction is completed. 1000mL, then the reaction system was extracted with 200mL of dichloromethane for several times, the organic phases were combined, dried over anhydrous sodium sulfate 50g, concentrated, and separated by silica gel column chromatography to obtain 19.04g of 3-bromobenzyltriazole; LC-MS ( ESI): m / z 237[M+H] + .
Embodiment 3
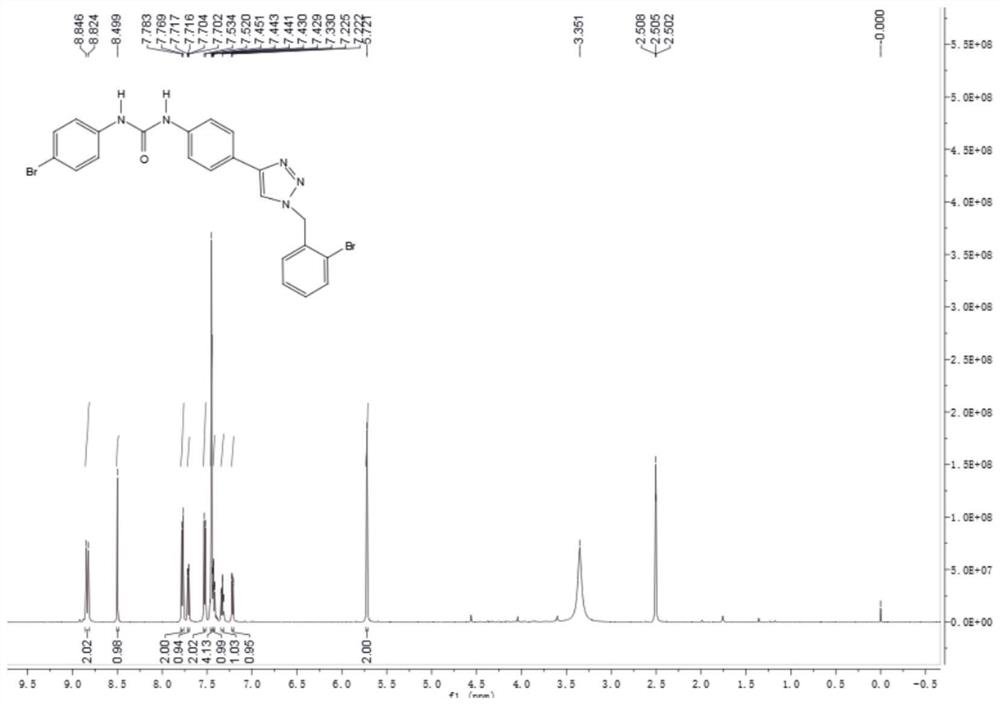
[0025]
[0026]In a reaction flask with nitrogen protection and temperature control device, add 25g of 2-bromobenzyl bromide into 400mL of acetonitrile, stir evenly, then cool down to 0°C, slowly add 250mL of acetonitrile dissolved with 10g of sodium azide dropwise, add dropwise After stirring for 1 hour, add 1.9 g of cuprous iodide and 300 mL of a tert-butanol solution in which 11 g of trimethylsilylacetylene was dissolved into the reaction system, stir to dissolve, slowly heat to 50°C, and add water to the reaction system after the reaction is completed. 1000mL, then the reaction system was extracted with 200mL of dichloromethane for several times, the organic phases were combined, dried over anhydrous sodium sulfate 50g, concentrated, and separated by silica gel column chromatography to obtain 17.12g of 2-bromobenzyltriazole; LC-MS ( ESI): m / z 237[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com