Preparation and amplification method and application of gamma delta T cell
A cell, in vitro amplification technology, applied in the field of cell biology, can solve the problem of unable to generate a sufficient number of cells, and achieve the effect of contributing to functional cure, strong proliferation ability, and strong killing of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
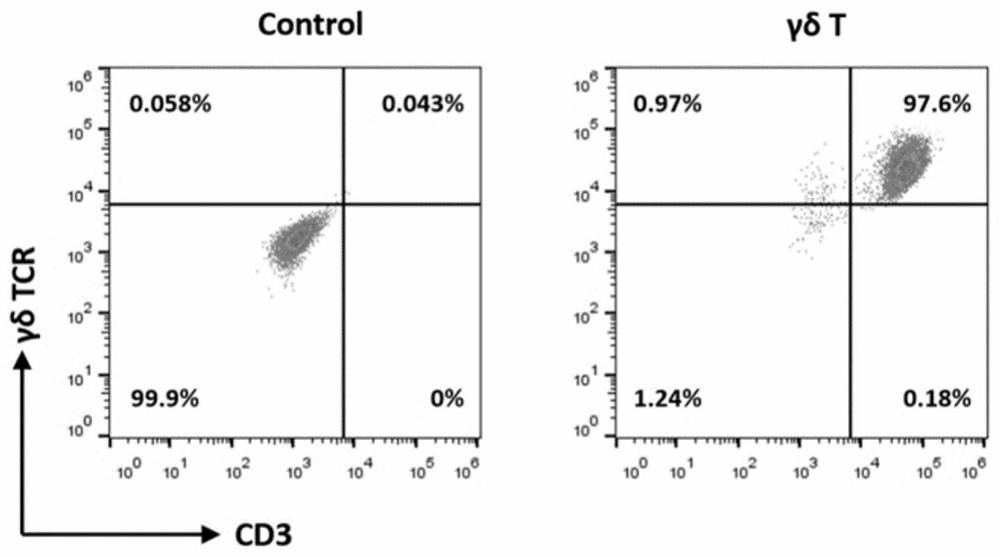
[0078] Example 1 Isolation of γδ T cells
[0079] Take the anticoagulated peripheral blood sample, after balancing, centrifuge at 20°C, 800×g for 20 minutes; transfer the upper plasma to a new sterile centrifuge tube. Use sterile phosphate buffered saline (PBS) 100ml to resuspend and dilute the cell pellet, and slowly attach the cell suspension to the human lymphocyte separation solution in stages (the volume ratio of cell suspension to human lymphocyte separation solution is 1:1: 1); Centrifuge at 20°C, 800×g for 20 minutes (1 increase, 0 decrease). Use a 10ml pipette to gently insert 0.5cm above the middle layer of thin cloud-like mononuclear cells, aspirate the layer of cells along the tube wall, transfer the middle layer of cells to a new 50ml sterile centrifuge tube, use 30ml sterile PBS was centrifuged at 20°C, 800 xg, for 20 minutes (1 9, 9 lower), and washed twice. Discard the supernatant, resuspend the cell pellet in 4ml x-vivo 15 medium, harvest PBMC, store at 4°C ...
Embodiment 2
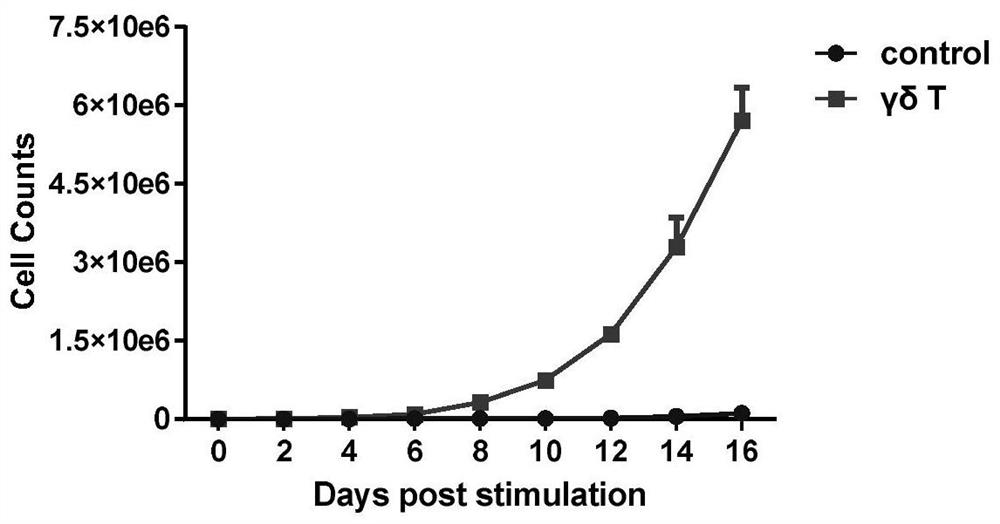
[0084] Example 2 γδT amplification
[0085] Use 25ml of the prepared γδT cell culture medium to resuspend the γδT cells obtained by magnetic bead sorting, transfer the cell suspension to a 75cm2 culture flask, and place the culture flask in a saturated humidity, 37°C, 5.0% CO2 incubator to cultivate. The growth of cells in the flasks was observed by microscope every other day. According to the cell growth state, observe the cell density in time and control it at 0.5-2×10 6 cells / ml, when the density exceeds this range, the medium is replaced with γδT cell culture medium, and the cell density is adjusted to within this range. After 14-16 days of continuous culture, the γδT cells were harvested, and the aseptic operation was observed during the entire cell culture process. Cell culture supernatants were taken at 0, 3, 7, 14, and 16 days of cell culture, and counted under a microscope to determine cell viability according to the above method; The total number and expansion fo...
Embodiment 3
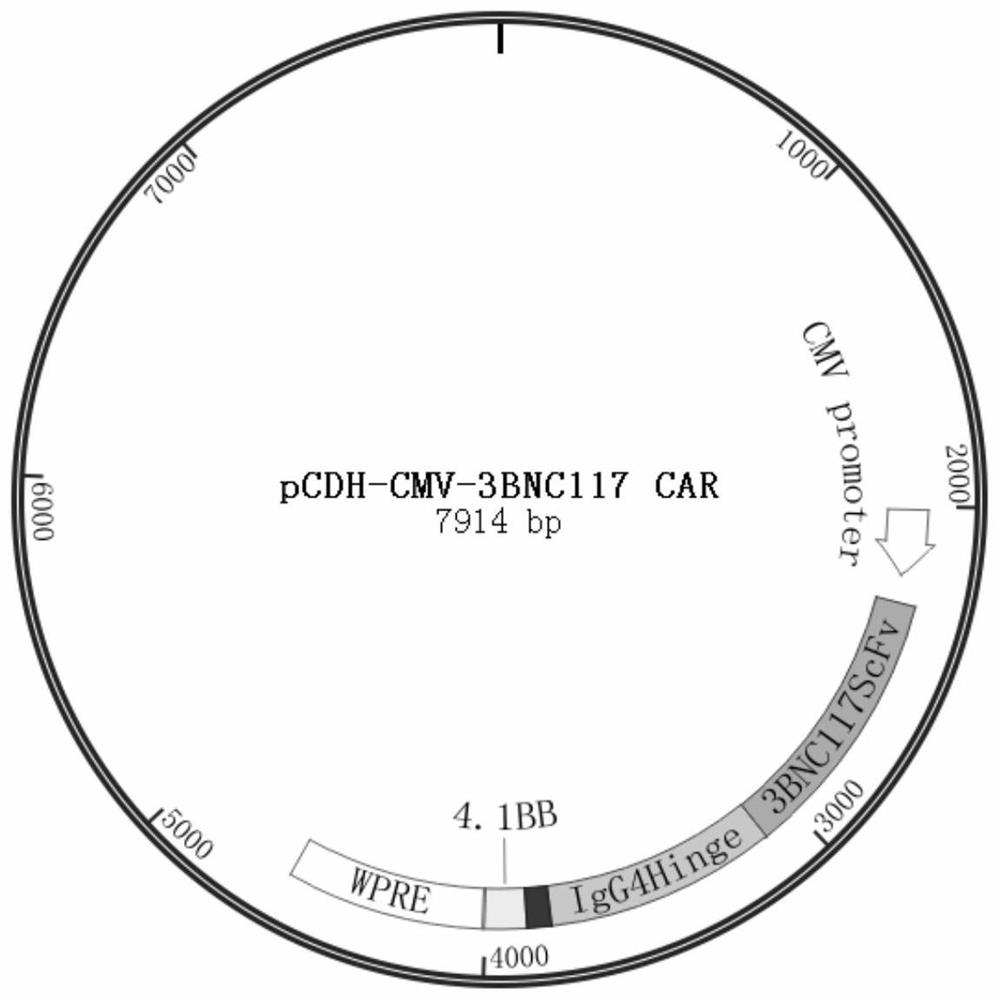
[0087] Example 3 In vitro construction of a chimeric antigen receptor expression vector targeting HIV-1 gp120
[0088] Using the pCDH-CMV-MCS-EF1α-Puro plasmid as the backbone, the MCS-EF1α-Puro fragment was removed by double digestion with EcoRI and SalI endonucleases. Then, using the pTRPE-3BNC117-G4H-BBz plasmid as a template, using onestep-3BNC117-F, onestep-3BNC117-R primers to PCR amplify ScFv, IgG4 hinge, CD8 molecules containing the variable region of HIV broadly neutralizing antibody Transmembrane region, 3BNC117 CAR fragment of 4-1BB. Finally, the double-enzyme digested product and the PCR product were gel recovered and then connected by Onestep homologous recombination. The ligated product was transformed in DH5α competent and then coated on Amp+ plate to screen positive clones. After the positive clones were expanded and cultured, the plasmids were extracted and sequenced for verification. The positive plasmid pCDH-CMV-3BNC117 ScFv-IgG4-CD8Tm-4-1BB was obtained an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com