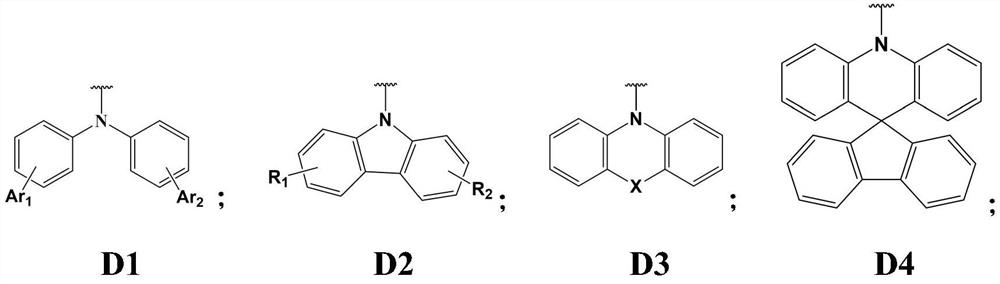
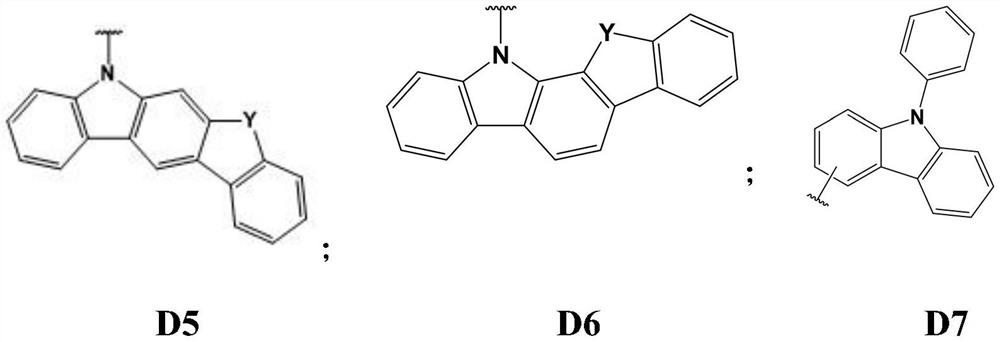
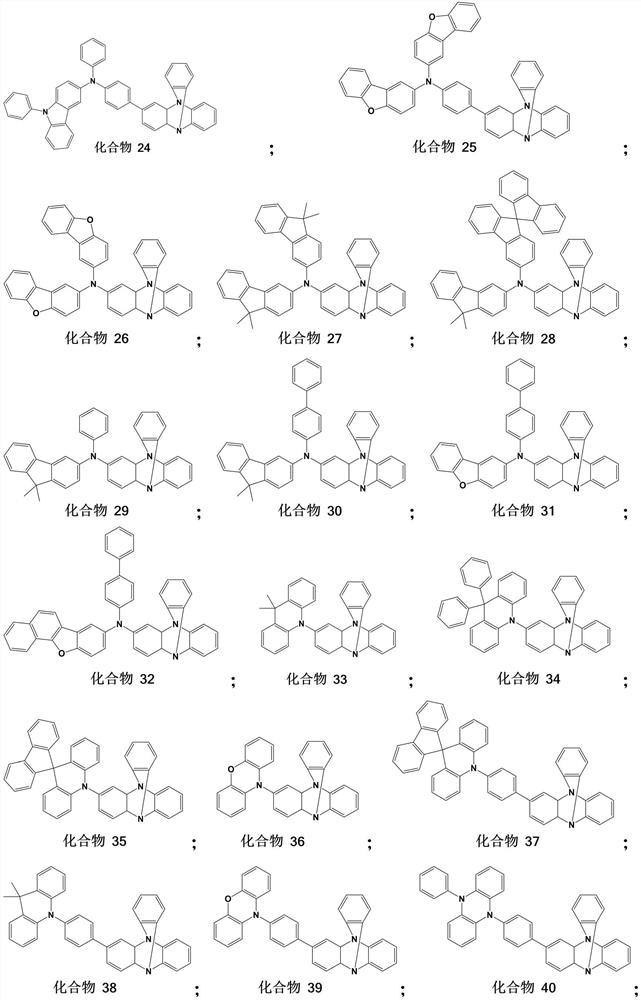
Tri (1, 2-phenyl) diamine derivative organic photoelectric material and application thereof
A technology of diamine derivatives and phenyl, which is applied in the field of organic electroluminescent materials, can solve the problems that the working life of materials cannot meet the application requirements, and achieve the effects of improving luminous stability, broad application prospects, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example provides the specific synthesis method of the specific compound involved in the tris(1,2-phenyl)diamine derivative and the corresponding intermediate.
[0036] (1) Synthesis of Intermediate 1
[0037]
[0038] 300g of intermediate 1-1, 1330.0g of intermediate 1-2, 929.6g of sodium tert-butoxide, and 8L of toluene were successively added to the three-necked flask, and 38.2g of cuprous iodide was added after the air in the reaction flask was replaced by nitrogen. The reaction solution was heated to 110°C under reflux and stirred for 4 hours. TLC monitored the complete consumption of the raw material intermediate 1-1 and then stopped heating. After cooling to room temperature, the reaction solution was washed with water until neutral. The organic phase was dried with anhydrous sodium sulfate and purified by silica gel column. 149.8 g of intermediate 1 were obtained with a yield of 27.8%.
[0039] (2) Synthesis of Intermediate 1
[0040]
[0041]In the ...
Embodiment 2
[0103] In this example, T1 energy, HOMO and LUMO energy level tests are respectively performed on some compounds involved in the present invention and existing materials.
[0104] The triplet energies (T1) of the highest molecularly occupied orbital (HOMO) and the lowest molecularly unoccupied orbital (LUMO) are the data obtained from the simulation calculations, and the experimental results are shown in Table 1.
[0105] Table 1. Performance test of tris(1,2-phenyl)diamine derivatives and existing materials
[0106]
[0107]
[0108] As can be seen from Table 1, the tris(1,2-phenyl)diamine derivatives of the present invention have higher triplet energy and more suitable HOMO / LUMO, which are beneficial to carrier transport and energy in OLED devices The transfer of these compounds can be used as hole transport materials, can also be used as light-emitting materials. Without specific limitation, the above-mentioned organic electroluminescent device may be a phosphorescen...
Embodiment 3
[0110] This example takes part of the tris(1,2-phenyl)diamine derivatives provided by the present invention as an example, and applies them to organic electroluminescent devices as light-emitting materials or hole-transporting materials to verify the obtained Excellent effect.
[0111] Specifically, the excellent effect of the OLED material of the present invention applied in the device is described in detail through the device performance of Device Examples 1-15 and Comparative Example 1. Examples 1 to 15 of the device of the present invention and Comparative Example 1 have exactly the same manufacturing process, and use the same glass substrate and electrode material, and the film thickness of the electrode material is also the same, the difference is the light-emitting layer or the second hole transport layer. Adjustments have been made as follows.
[0112] Device Example 1
[0113] This embodiment provides an organic electroluminescence device, the structure of which is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com