Ambroxol hydrochloride injection and preparation method thereof
A technology of ambroxol hydrochloride and injection, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of liposomes containing impurities, low stability, easy oxidation, etc. problem, to achieve the effect of increasing hydrophobicity, ensuring stability, and a single component
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
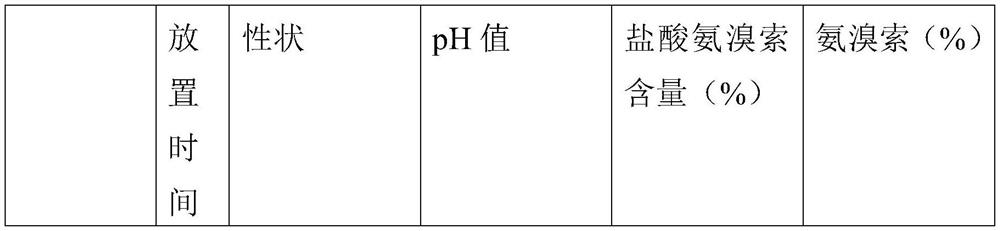
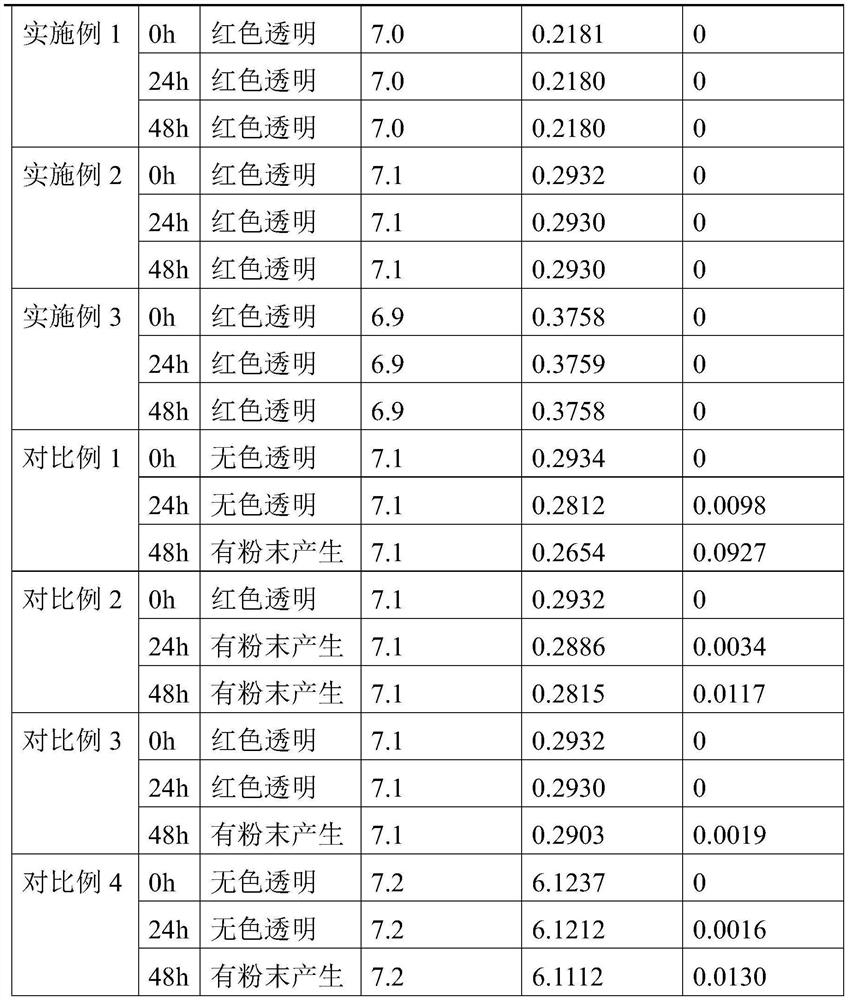
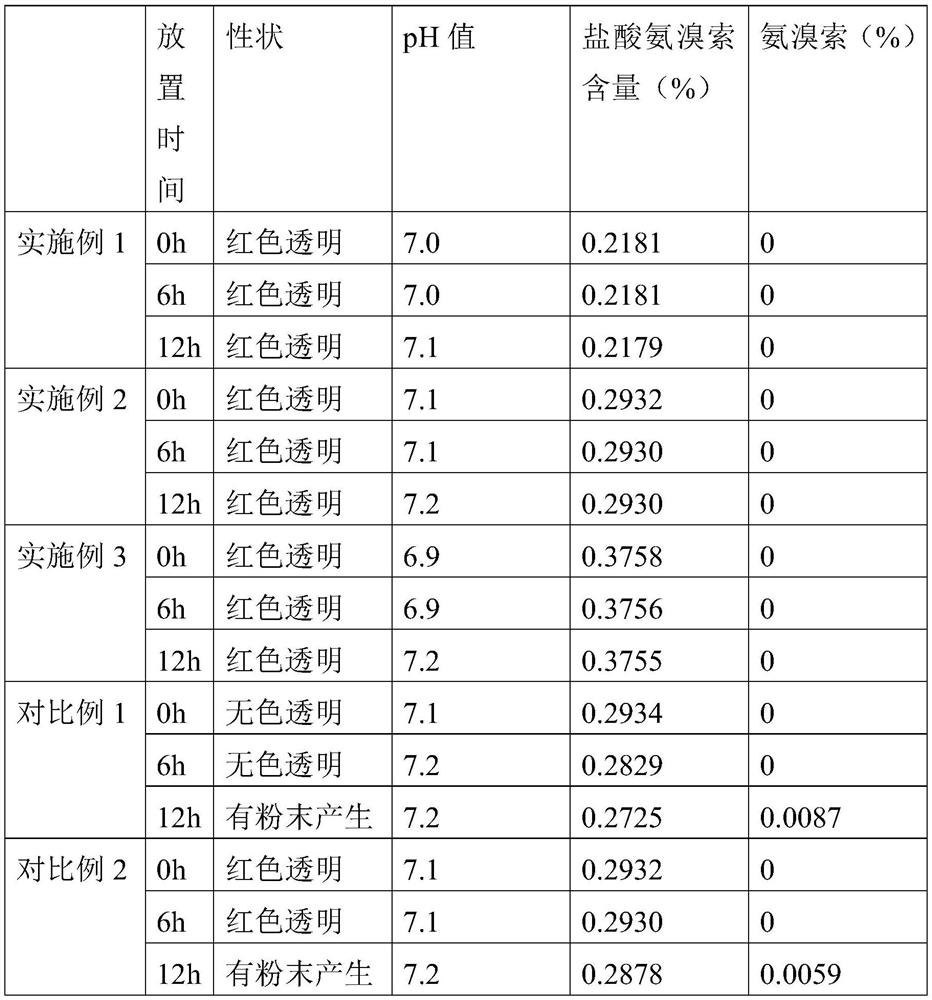
[0022] A kind of ambroxol hydrochloride injection, comprising the following raw materials by weight: 2 parts of ambroxol hydrochloride, 4 parts of dipalmitoyl phosphatidylethanolamine, 1 part of cholesterol, 0.5 parts of astaxanthin, 1.5 parts of disodium hydrogen phosphate, ascorbic acid 1 part, 7 parts sodium chloride and 900 parts water.
Embodiment 2
[0024] A kind of ambroxol hydrochloride injection, comprising the following raw materials by weight: 3 parts of ambroxol hydrochloride, 5 parts of dipalmitoyl phosphatidylethanolamine, 1 part of cholesterol, 0.8 parts of astaxanthin, 2 parts of disodium hydrogen phosphate, ascorbic acid 1.5 parts, 10 parts sodium chloride and 1000 parts water.
Embodiment 3
[0026] A kind of ambroxol hydrochloride injection, comprising the following raw materials by weight: 5 parts of ambroxol hydrochloride, 6 parts of dipalmitoyl phosphatidylethanolamine, 2 parts of cholesterol, 1 part of astaxanthin, 2.5 parts of disodium hydrogen phosphate, ascorbic acid 2 parts, 12 parts sodium chloride and 1300 parts water.
[0027] The preparation method of above-mentioned a kind of ambroxol hydrochloride injection, comprises the following steps:
[0028] (1) mix disodium hydrogen phosphate, ascorbic acid, sodium chloride and water to make buffer solution;
[0029] (2) adding dipalmitoyl phosphatidylethanolamine, cholesterol and astaxanthin into the buffer solution, and ultrasonically dispersing to make an empty liposome solution;
[0030] (3) mixing ambroxol hydrochloride and astaxanthin again and adding to the empty liposome solution, and sterilizing after ultrasonic dispersion to obtain ambroxol hydrochloride injection.
[0031] The pH range of the buff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com