Cetirizine hydrochloride tablet and preparation method thereof
A technology of cetirizine hydrochloride and rizine tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as adhesion and agglomeration, and shelf-life content decline , to achieve the effect of being suitable for industrial production, simple preparation process and excellent detection stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Cetirizine Hydrochloride Tablets
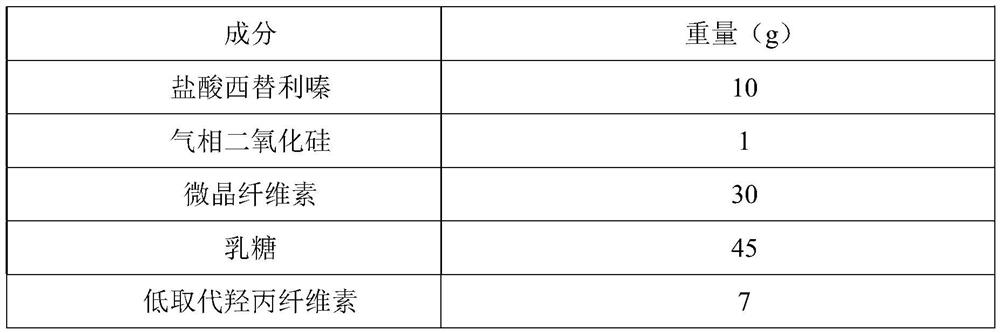
[0029] The prescription consists of 1000 tablets
[0030]
[0031]
[0032] Preparation Process:
[0033] (1) batching process: Cetirizine hydrochloride and fumed silica are pulverized using a 120-mesh bottom mesh;
[0034] (2) Mixing and sieving process: in the mixing and sieving process, the order of adding the raw and auxiliary materials is microcrystalline cellulose, cetirizine hydrochloride fumed silica powder, lactose, low-substituted hypromellose, and the mixing time is 5 minutes. 30 mesh sieve, mix for another 10 minutes, and then mix for 3 minutes with magnesium stearate;
[0035] (3) Tabletting process: using a rotary tablet press, tableting; the tableting speed is 150,000 tablets / hour, and the average tableting main pressure is 15.0KN;
[0036] (4) Coating process: in the coating process, the film-coating premix is added to purified water, stirred for 30 minutes to prepare a suspension coating solution ...
Embodiment 2
[0037] Example 2: Cetirizine Hydrochloride Tablets
[0038] The prescription consists of 1000 tablets
[0039] Element Weight (g) cetirizine hydrochloride 10 Fumed silica 3 microcrystalline cellulose 50 lactose 65 low substituted hydroxypropyl cellulose 13 Magnesium stearate 3 Opadry 7
[0040] Preparation Process:
[0041] Same as Example 1.
Embodiment 3
[0042] Example 3: Cetirizine Hydrochloride Tablets
[0043] The prescription consists of 1000 tablets
[0044]
[0045]
[0046] Preparation Process:
[0047] Same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com