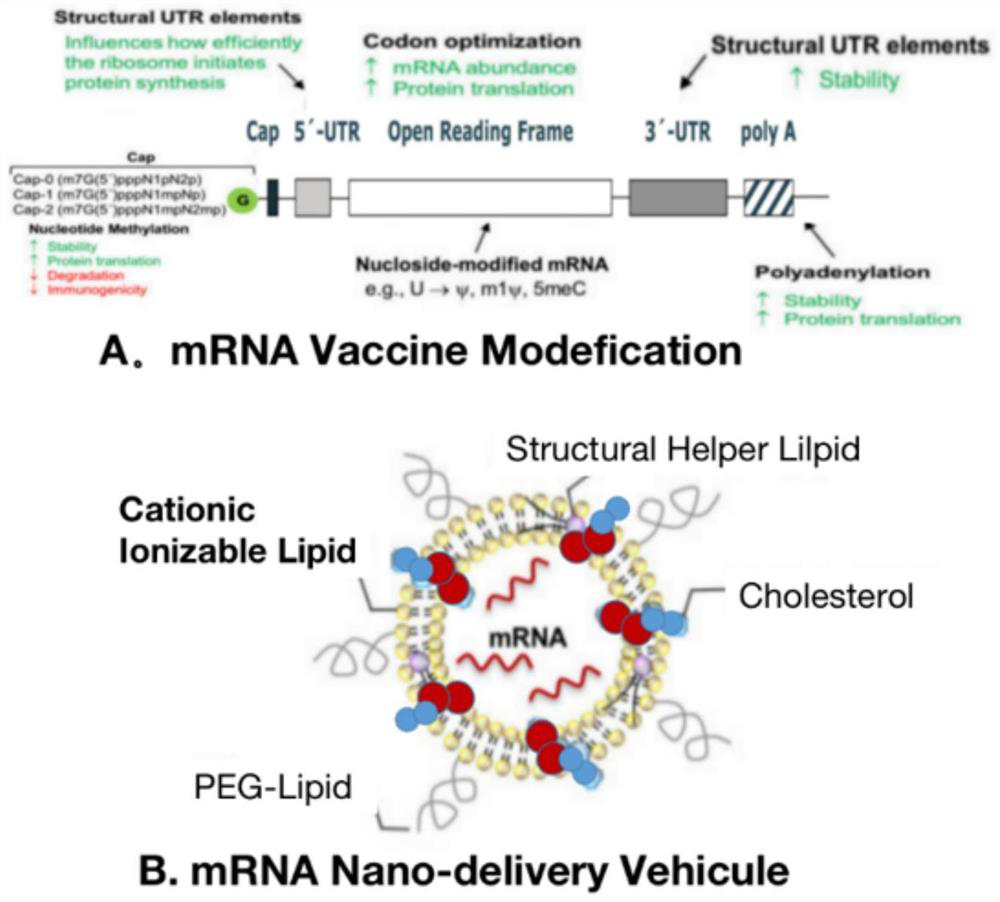
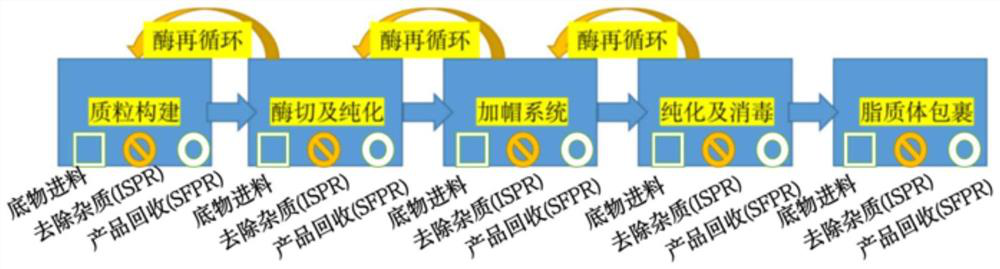
New coronavirus wild type and variant combined vaccine based on mRNA (messenger ribonucleic acid) and preparation method thereof
A combined vaccine and virus mutation technology, which is applied in the field of vaccine development and biomedicine, can solve the problems of poor effect, achieve the effects of reducing costs, flexible cost-effectiveness, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] This example is used to illustrate the correctness and effectiveness of the novel combination vaccine sequence designed by the present invention, and also to verify that the mutant strain has stronger binding ability to receptors and is more infectious.
[0091] First, the pseudovirus produced with this sequence binds to the ACE2 receptor and is verified. For this purpose, pseudotyped lentiviral particles with different spike proteins were made. Briefly, pseudoviruses were generated in 293T cells by PEI transfection of the lentiviral backbone encoding cmv-luciferasi-ires-zsgreen, along with lentiviral helper plasmids and mutant expression plasmids for each spike; collected in 293T-ACE2 cells And after filtration, run quantitatively by flow cytometry titration.
[0092] (1) Method
[0093] Flow cytometry titers were used to determine the infectious units and infection rates of the SARS-CoV-2 pseudovirus.
[0094] 400,000 293T-ACE2 cells were seeded in each well of a 1...
Embodiment 2
[0099] In this example, a variety of novel combination vaccine wild-type and mutant strain antigen expression plasmids are designed and constructed to illustrate the intracellular expression of the novel combination vaccine wild-type and mutant strain antigens of the present invention.
[0100] (1) Method
[0101] SARS-CoV-2 S protein wild type (Swild), S protein mutant (Somicron), RBD wild type (RBDw), RBD mutant (RBDomi), S protein wild type + S protein mutant (Sw+Somi), S Protein wild type + RBD mutant (Sw + RBDomi), S protein mutant + RBD wild type (Somi + RBDw) vaccine proteins, produced in suspension cultured Expi293F cells using Expi293F expression medium (Life Technologies), temperature 33 ℃, humidity 70%, 8% CO 2 , rotating speed 150rpm; use PEI-MAX (Polyscience) to transfect the cultured cells, the cell density is 3 million cells / mL, and culture for 3 days; centrifuge to clarify the supernatant (4000rcf, 5min), add PDADMAC solution to the final concentration of 0.03...
Embodiment 3
[0108] In this example, the expression level and duration in mice after inoculation of the mRNA vaccine of the present invention are studied to illustrate the speed and duration of the rise in immunity in vivo.
[0109] (1) Method
[0110] The above-mentioned wild-type and mutant DNA fragments of the new coronavirus and the plasmid vector encoding the DNA sequence of luciferase were linearized and transcribed in vitro to synthesize mRNA-LNP vaccine. The mRNA vaccine prepared above was injected into mice, and the in vivo fluorescein detection was performed according to the observation time.
[0111] To examine the in vivo distribution and duration of FLuc mRNA-LNPs, 6-week-old female BALB / c mice (n=36) were inoculated with 10 μg of FLuc mRNA- LNP. At 6h, 12h, 24h, and 2 to 7 days after inoculation, animals were injected intraperitoneally with luciferase substrate (Promega). After 3 min of reaction, the fluorescence signal was collected by IVIS spectrometer (PerkinElmer) for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com