Preparation method and application of hylagolil intermediate 1 and hylagolil intermediate 2
A technology of elagolix and intermediates, which is applied in the field of compound preparation, can solve the problems of difficult refining, difficult refining, unfavorable scale-up production, etc., and achieve the effects of guaranteed purity, simple synthesis and operation, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] This embodiment provides a preparation method of Elagoli Intermediate 2.
[0032] 1. Preparation of intermediate 1 crude product:
[0033]
[0034] Intermediate 6 (50 g, 117 mmol) and D-Boc-phenylglycinol mesylate (59 g, 188 mmol) were dissolved in 300 mL of DMF, potassium carbonate (41 g, 293 mmol) was added under stirring, and the temperature was raised to 40 ± 5 °C for reaction 16h, stop heating;
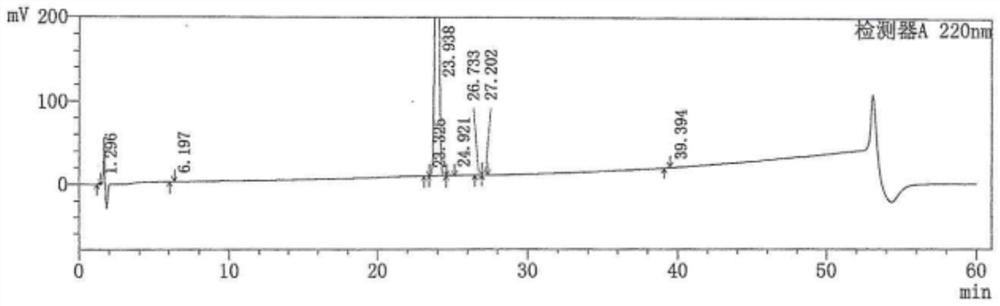
[0035] The reaction solution was concentrated to about 200g, heated to 60±5℃, slowly added 800mL of water and stirred to dissolve, continued to grow crystals for 0.5h, slowly cooled to room temperature, continued to stir for 1h, and the filter cake was washed with 100mL of water; dried at 50℃ for 4- 8h, the crude product of Intermediate 1 was obtained, weighing 100.5g.
[0036] 2. Purification of crude intermediate 1
[0037] Take 100 g of the crude intermediate 1 and add it to 300 mL of ethanol, heat to 60±5°C, stir to dissolve, add 10 mL of water and continue to st...
Embodiment 2
[0041] This embodiment provides a purification method of Elagoli Intermediate 1.
[0042] 1. Preparation of crude intermediate 1: the same as in Example 1.
[0043] 2. Purification of crude intermediate 1
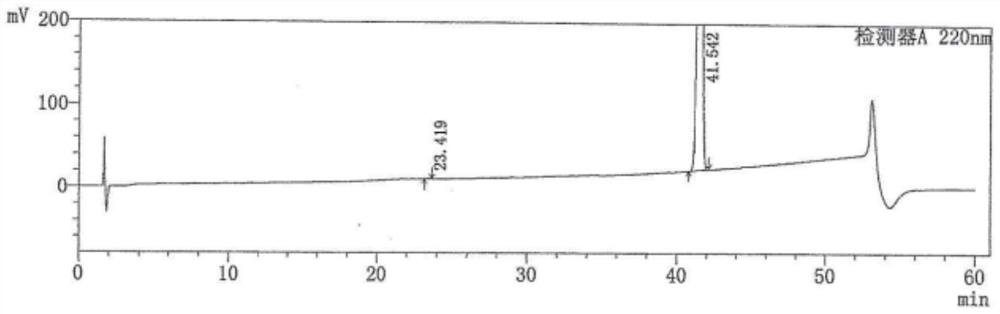
[0044] Take 10 g of the above-mentioned crude intermediate 1 and add it to 30 mL of ethanol, heat to 60 ± 5 °C, stir to dissolve, add 5 mL of water and continue to stir for 30 min, add 0.1 g of seed crystals (that is, intermediate 1 crystals); Slowly cool down to 10 ± 5°C, stirring was continued overnight. The next day was filtered, and the filter cake was washed with 10 mL of water; dried at 50° C. to obtain 6.3 g of a white solid with a yield of 84.2% and a purity of 99.46%.
Embodiment 3
[0046]This example provides a purification method of Elagoli Intermediate 1.
[0047] 1. Preparation of crude intermediate 1: the same as in Example 1.
[0048] 2. Purification of crude intermediate 1
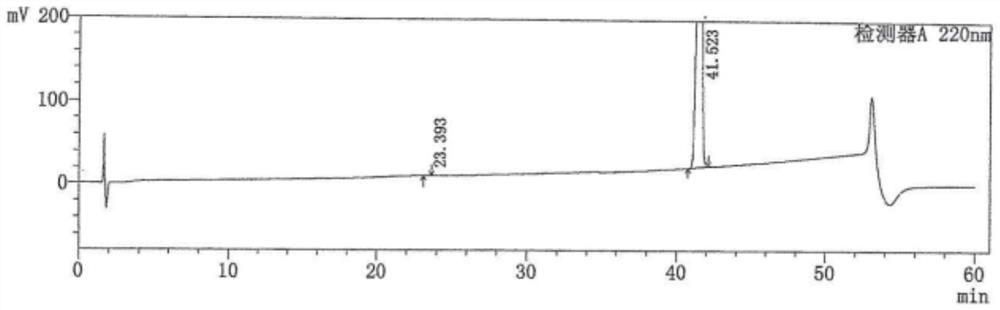
[0049] Add 10 g of the crude intermediate 1 to 30 mL of tetrahydrofuran, heat to 60 ± 5 °C, stir to dissolve, add 60 mL of water and continue stirring for 30 min; slowly cool down to 20 ± 5 °C, continue stirring overnight. The next day, filtered, and the filter cake was washed with 10 mL of water; dried at 50° C. to obtain 6.6 g of a white solid with a yield of 87.3% and a purity of 99.13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com