Application of flavonoid compound to preparation of beta-lactamase inhibitor
A technology of flavonoids and lactamase, which is applied in the field of serine β-lactamase inhibitors, can solve the problems of high price and few types of inhibitors, achieve good inhibitory activity and reduce the difficulty of obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
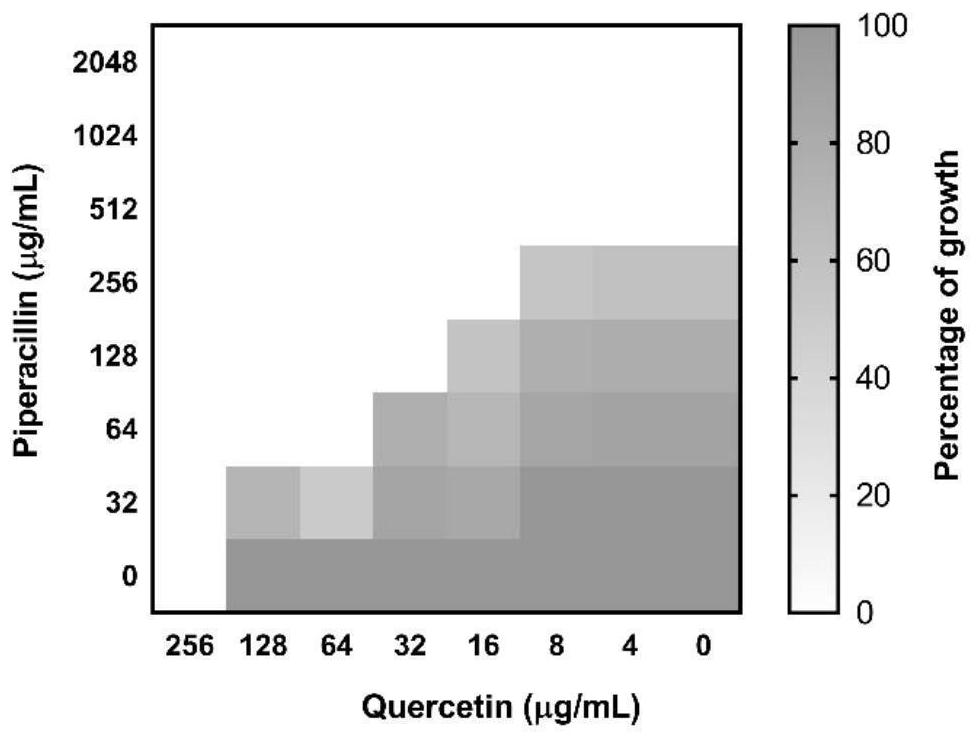
[0042] Embodiment 1: a kind of antibacterial drug composition
[0043] This embodiment discloses an antibacterial drug composition, the composition contains a serine β-lactamase inhibitor and a β-lactam antibiotic, and the serine β-lactamase inhibitor is a flavonoid compound.
[0044] The flavonoid compound described in this example is a combination of quercetin, 3',4',7-trihydroxyflavonoid, fisetin and luteolin.
Embodiment 2
[0045] Example 2: A method for designing the structure of an OXA-48 inhibitor
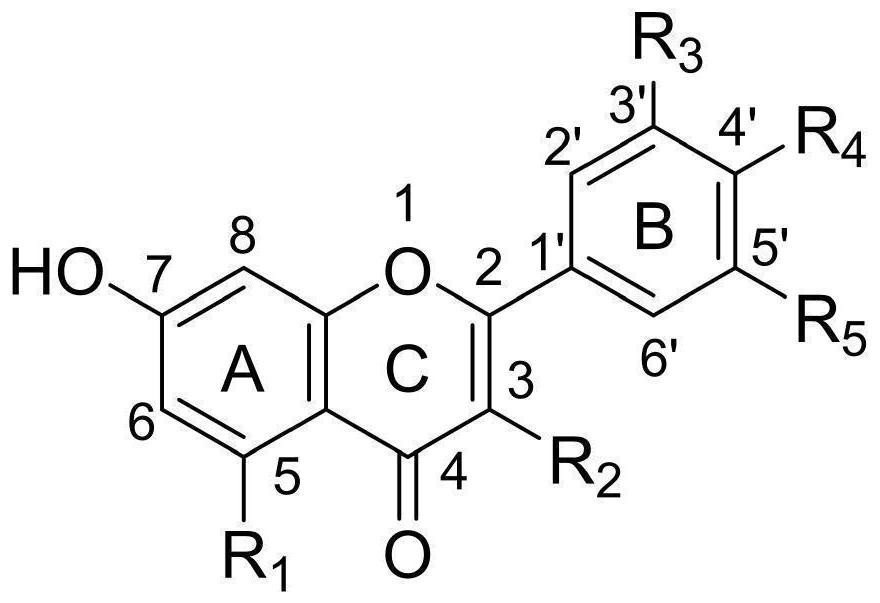
[0046] The present embodiment provides a method for designing the structure of an OXA-48 inhibitor, which has designed a substance conforming to the following general structural formula:
[0047]
[0048] In the above formula: the 2 and 3 positions contain a double bond; the R1, R2, R3, R4 and R5 are hydroxy substances, and the OXA-48 inhibitory activity of the synthesized compound is measured.
Embodiment 3
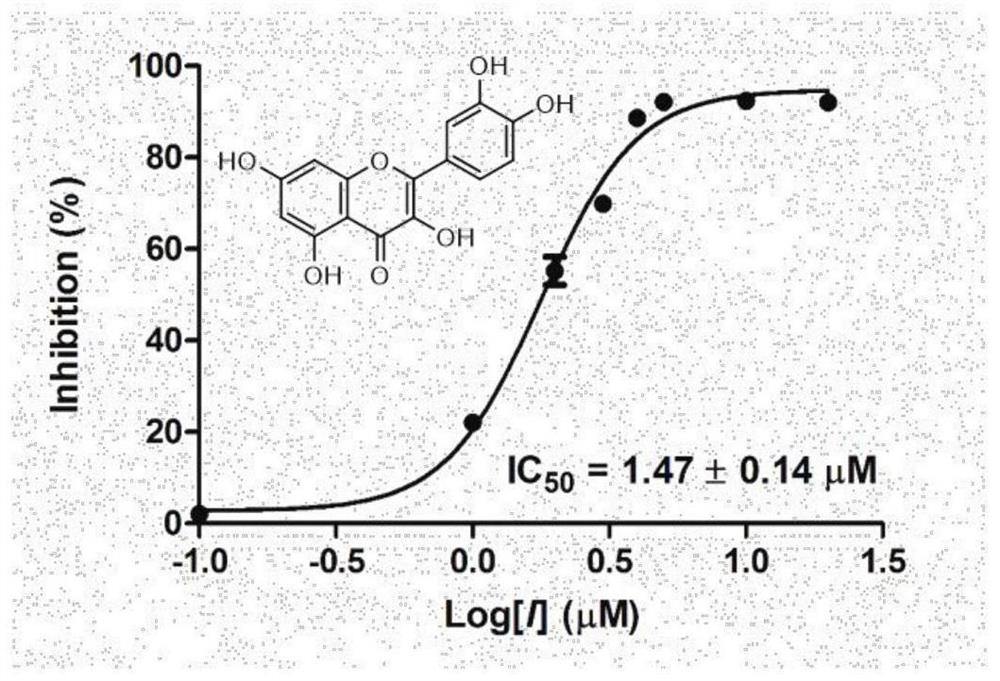
[0049] Example 3: Determination of the inhibitory activity of quercetin and its structural analogs on OXA-48 (IC 50 )
[0050] The change of absorbance after OXA-48 serine β-lactamase hydrolyzed the substrate was monitored at a wavelength of 495 nm using cefnitrothiophene as the hydrolysis substrate. Assay buffer is 0.1M PBS (pH 7.0), 30mM NaHCO 3 , the temperature is 25 ℃. The specific method is as follows: incubate the enzyme and inhibitor in the buffer for 30 minutes, so that the inhibitor and the enzyme can fully interact, add the mixture to a 96-well plate, and immediately use a microplate reader to record the absorbance change before the reaction 30s after adding the substrate. Calculate the initial reaction rate. The concentration of substrate and enzyme was kept constant throughout the experiment, the concentration of inhibitor was changed, the percentage inhibition rate at different inhibitor concentrations was calculated, and the IC was calculated by nonlinear fit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com