Application of compound in preparation of antiviral drug and application
A technology of antiviral drugs and compounds, applied in the field of medicine, can solve the problems such as the inability to provide technical support, research reports, and no antiviral drugs for new antiviral clinical candidates, so as to expand the range of options, relieve pulmonary inflammation, and relieve Effects of viral pneumonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Compound N-[(2-methoxyphenyl)methyl]-2-[4-(4-methoxyphenyl)-2-oxybenzopyran-7-yl] provided in the examples of the present invention Oxyacetamide, the molecular structure is as follows:
[0032]
[0033] The compound N-[(2-methoxyphenyl)methyl]-2-[4-(4-methoxyphenyl)-2-oxybenzopyran-7-yl]oxyacetamide is in Use in the preparation of anti-coronavirus drugs.
[0034] In the embodiment of the present invention, the compound N-[(2-methoxyphenyl)methyl]-2-[4-(4-methoxyphenyl)-2-oxybenzopyran-7 The dosage of -yl]oxyacetamide is 0.001g / kg / d~0.009g / kg / d.
Embodiment 2
[0036] N-[(2-methoxyphenyl)methyl]-2-[4-(4-methoxyphenyl)-2-oxybenzopyran-7-based on the compound provided in Example 1 of the present invention yl]oxyacetamide uses such as figure 1 As shown, the examples of the present invention provide a compound N-[(2-methoxyphenyl)methyl]-2-[4-(4-methoxyphenyl)-2 for verifying the use -The construction method of the animal model of the efficacy of oxybenzopyran-7-yl]oxyacetamide includes:
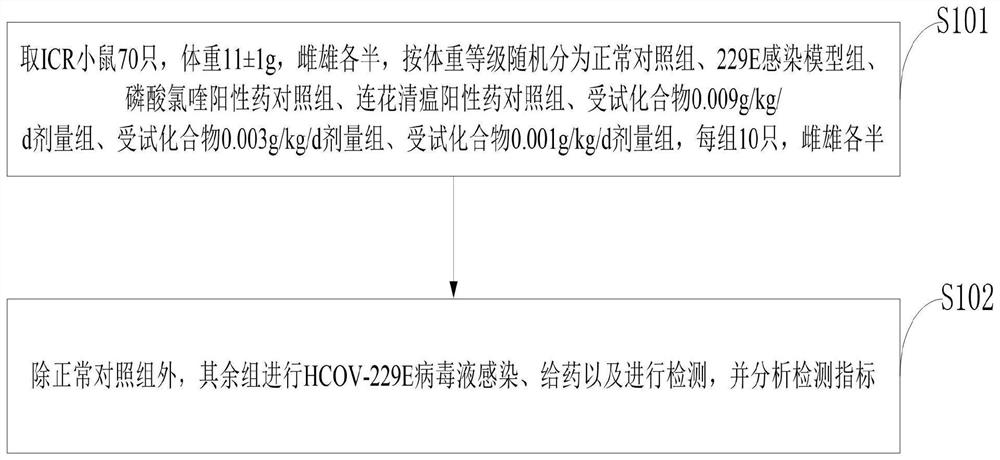
[0037] S101, 70 ICR mice, weighing 11±1 g, half male and half male, were randomly divided into normal control group, 229E infection model group, chloroquine phosphate positive drug control group, Lianhua Qingwen positive drug control group, and control group according to body weight. Test compound 0.009g / kg / d dose group, test compound 0.003g / kg / d dose group, test compound 0.001g / kg / d dose group, 10 in each group, half male and half male;
[0038] S102, except for the normal control group, the other groups were infected with HCOV-229E virus liquid, ad...
Embodiment 3
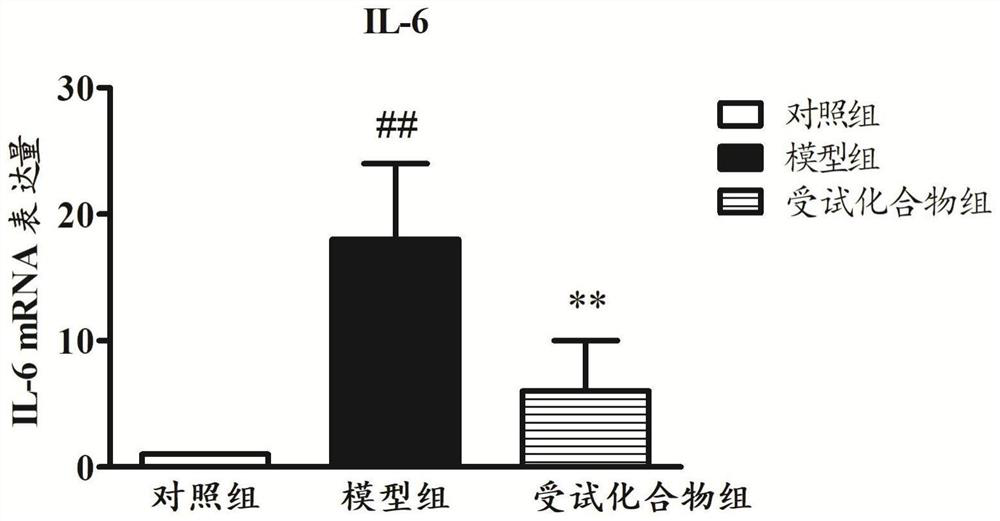
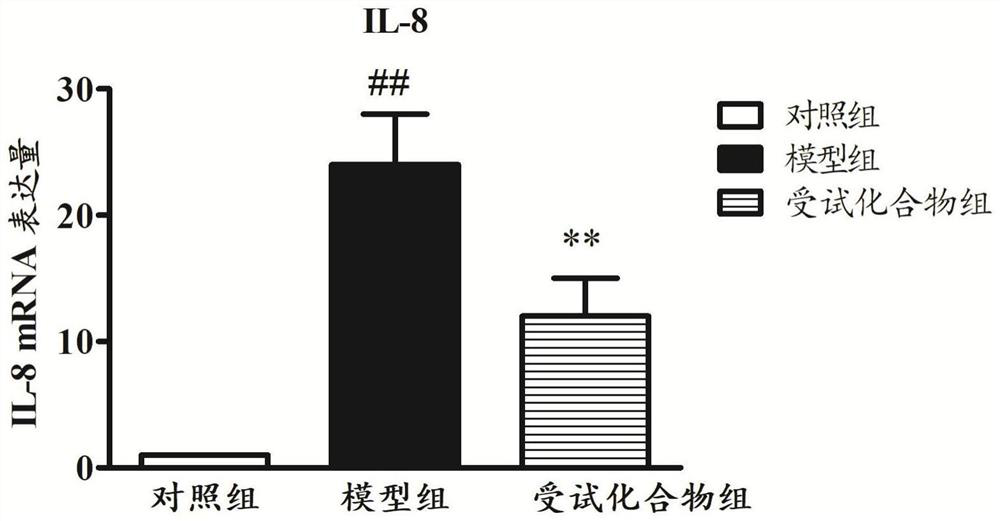
[0040] Based on the construction method of the animal model provided by the embodiment of the present invention, the detection index movement includes:
[0041] Lung index (%) = wet lung weight (g) × 100 / body weight (g);
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com