Marker and application thereof in preparation of product for evaluating immune function of organism
A marker and product technology, applied in the application field of the product, can solve the problems of large antibody reagents and blood sample volume, time-consuming, limited promotion and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
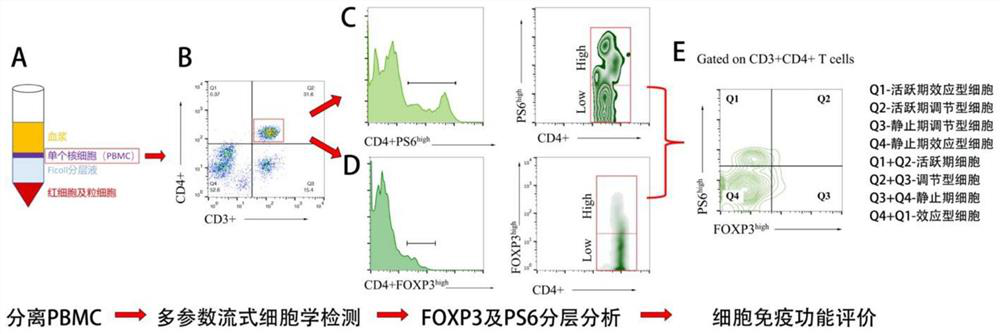
Image
Examples
Embodiment 1
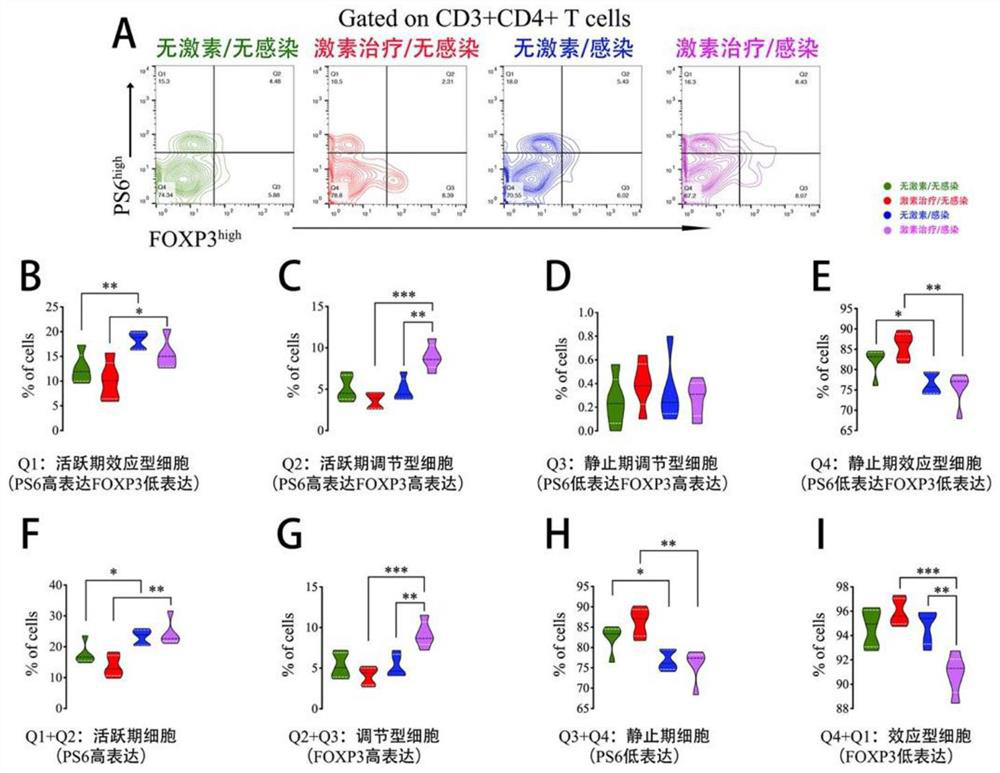
[0049]Glucocorticoid (GC) is a landmark drug in the medical field. It is widely used in all clinical specialties and plays an irreplaceable role in the treatment of connective tissue diseases, inflammation, allergy and other systemic diseases. Taking GC for the treatment of nephrotic syndrome (NS) as an example, the conventional treatment regimen for GC requires a large initial dose, a long maintenance course and a slow dose reduction process. Although this improves the efficacy of GC, it is also prone to significant adverse drug reactions due to the large cumulative dose of GC. Among them, the low resistance of the body caused by the extensive immunosuppressive effect of GC, which eventually leads to severe infection that endangers the patient's life, is the most serious complication. This occurs frequently in patients with NS. A single reduction in GC dose and duration is currently the only feasible way to reduce risk, but this in turn may reduce the efficacy of GC in treat...
Embodiment 2
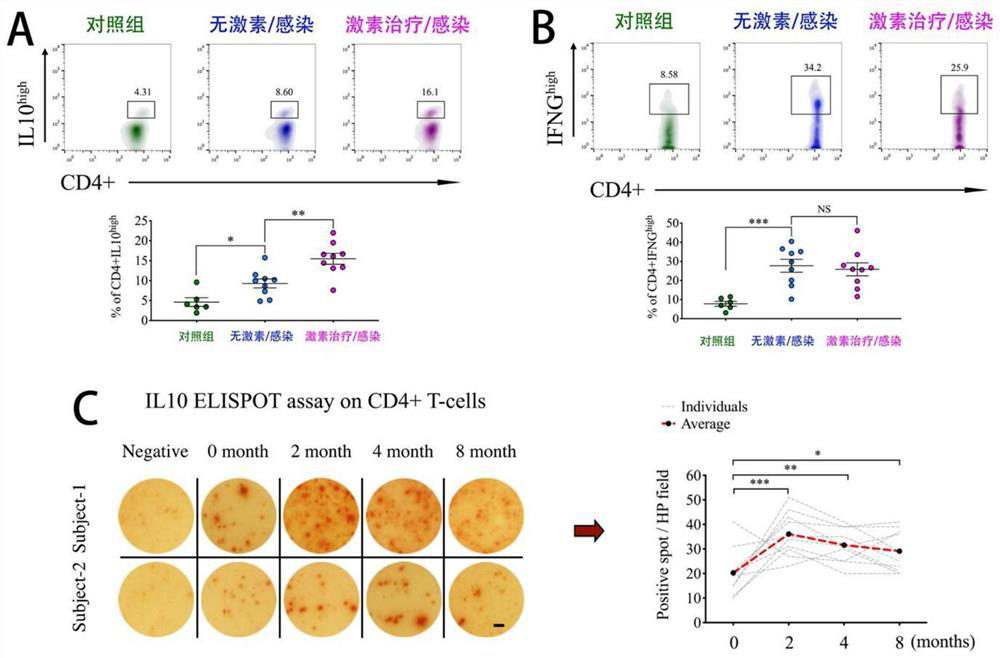
[0052] In order to further verify the molecular mechanism of the above clinical results, the inventors detected the expression levels of anti-inflammatory factors (IL10) and inflammatory factors (IFNG) in peripheral CD3+CD4+ T cells of patients under different conditions. The expression level of anti-inflammatory factor IL10 affects the immune function of cells under infection ( image 3 A), which has less interference with the expression of inflammatory factor IFNG ( image 3 B). The inventors further isolated CD4+ T cells from patients at different stages of GC treatment and conducted in vitro antigen stimulation experiments, which also confirmed that GC-treated CD3+ CD4+ T cells secreted higher levels of IL10 after antigen stimulation; The high expression state persisted during 8 months of GC reduction ( image 3 C). The current research results agree that regulatory CD4+ T cells with high expression of FOXP3 are the main cells that produce IL10. Therefore, the above in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com