Preparation method of BTK kinase inhibitor key intermediate
A technology of intermediates and reagents, applied in the field of medicine, can solve problems such as poor amplification feasibility, difficult purification, and low total yield, and achieve the effects of reducing pollution, simple reaction operation, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] first step:
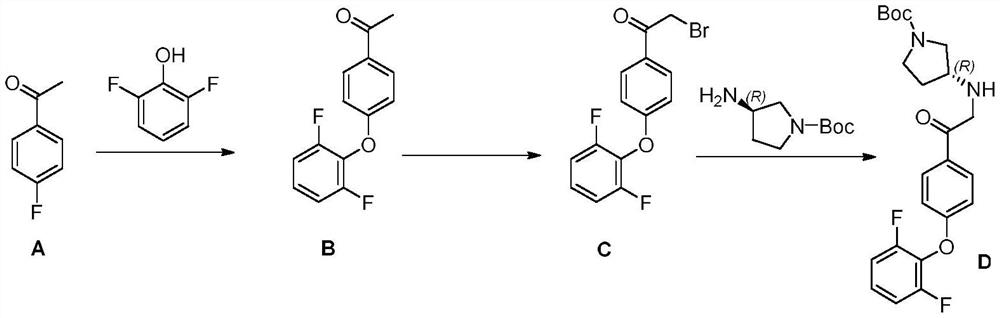
[0044] Under nitrogen protection, N,N-dimethylformamide 10kg, 2,6-difluorophenol (0.85kg, 6.53mol) and p-fluoroacetophenone (1kg, 7.24mol) were added successively in the reactor, stirring Wait for the system to dissolve. Next, sodium carbonate (1.53 kg, 14.48 mol) was added, and the temperature of the oil bath was raised to 150-155° C. and stirring was continued for 16 hours. After the detection reaction was completed, the material was cooled to 60° C., poured into 10 L of ice water, filtered and washed to obtain 1.46 kg of Intermediate B with a yield of 90.2%. Step 2:
[0045] Under nitrogen protection, in the reactor, intermediate B (1.46kg, 5.88mol) and glacial acetic acid 0.50kg were added in dioxane 15L, and the reaction was stirred after adding dibromohydantoin (0.86kg, 3.00mol) in 3 batches Overnight, the reaction was poured into 15 L of water with stirring at room temperature, filtered, and recrystallized to obtain 1.61 kg of Intermediate C, wit...
Embodiment 2
[0054] The first three steps are the same as those in Example 1.
[0055] the fourth step:
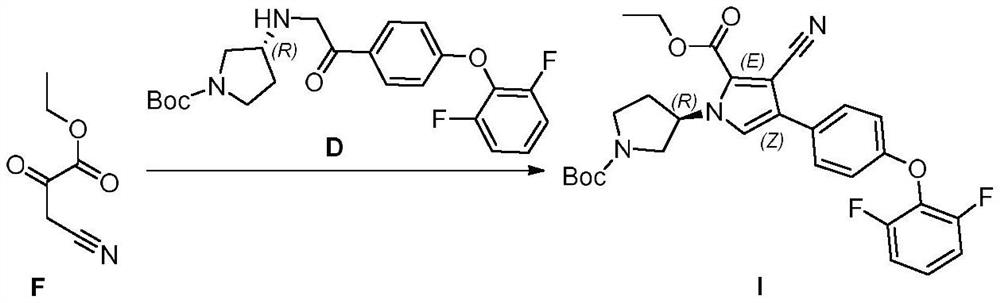
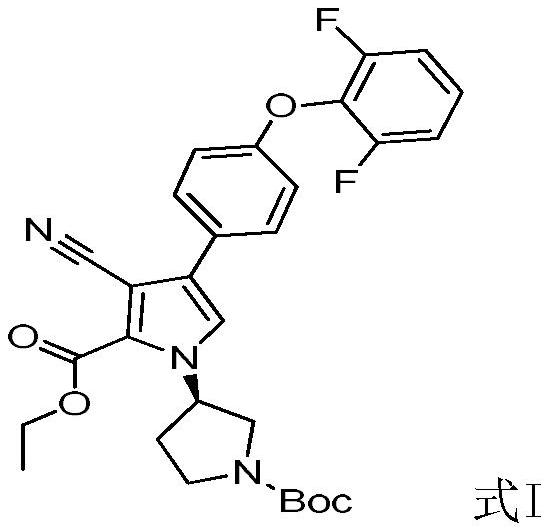
[0056] A 2.5M n-butyllithium / n-hexane solution (20L, 50mol) was cooled to -60 to -78°C, and acetonitrile (2.05kg, 50mol) was slowly added dropwise. After the mixture was stirred at -60 to -78°C for 1 hour, ethyl oxalate (7.30 kg, 50 mol) / n-hexane (21 L) solution was slowly added dropwise. After the mixture was stirred at -60 to -78°C for 1 hour, the dry ice bath was removed and slowly returned to ambient temperature. The mixture was poured into 70 L of ice water, and extracted three times with methyl tert-butyl ether. The aqueous phase was cooled to 0°C and adjusted to pH=3-4 with 6M hydrochloric acid. It was extracted three times with ethyl acetate, and the organic phases were combined, dried and concentrated to obtain Intermediate F (5.74 kg, yield: 81.30%).
[0057] the fifth step:
[0058] Under nitrogen protection, in the reactor, add intermediate D (18.02kg, 41.66mol), inter...
Embodiment 3
[0060] The first three steps are the same as those in Example 1.
[0061] the fourth step:
[0062] 1M KHMDS / tetrahydrofuran solution (154L, 154mol) was cooled to -60~-78°C, and acetonitrile (6.31kg, 154mol) was slowly added dropwise. The mixture was stirred at -60 to -78°C for 1 hour, followed by the slow dropwise addition of a solution of ethyl oxalate (17.54 kg, 140 mol) / 2-methyltetrahydrofuran (50 L). After the mixture was stirred at -60 to -78°C for 1 hour, the dry ice bath was removed and slowly returned to ambient temperature. The mixture was poured into 180 L of ice water and extracted three times with methyl tert-butyl ether. The aqueous phase was cooled to 0°C and adjusted to pH=3-4 with 6M hydrochloric acid. It was extracted three times with ethyl acetate, and the organic phases were combined, dried and concentrated to obtain Intermediate F (16.36 kg, yield: 82.79%).
[0063] the fifth step:
[0064] Under nitrogen protection, in the reactor, add intermediate D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com