Metal-based ionic liquid catalyst for preparing polyester through coupling reaction as well as preparation method and application of metal-based ionic liquid catalyst
A technology of ionic liquid and coupling reaction, which is applied in the field of metal-based ionic liquid catalyst and its preparation, can solve the problems of unsatisfactory activity, stability, selectivity and dispersion, and achieve good thermal and chemical stability, absorption Enhanced electrical capacity, non-flammable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1. Mix 0.5 mol of imidazole with 0.5 mol of dichloromethane, react at 70° C. for 12 h, wash with ethyl acetate, spin-evaporate, and vacuum dry to obtain intermediate Cl-I, respectively.
[0046] Step 2. N 2 Under the protection, 0.25mol of benzoic acid was added to the reactor equipped with the above-mentioned 0.5mol of intermediate Cl-I, stirred and dissolved, condensed and refluxed, the reaction time was controlled at 5h, washed with acetonitrile after the reaction was completed, rotary evaporated, and vacuum dried Afterwards, different kinds of intermediate Cl-II were obtained.
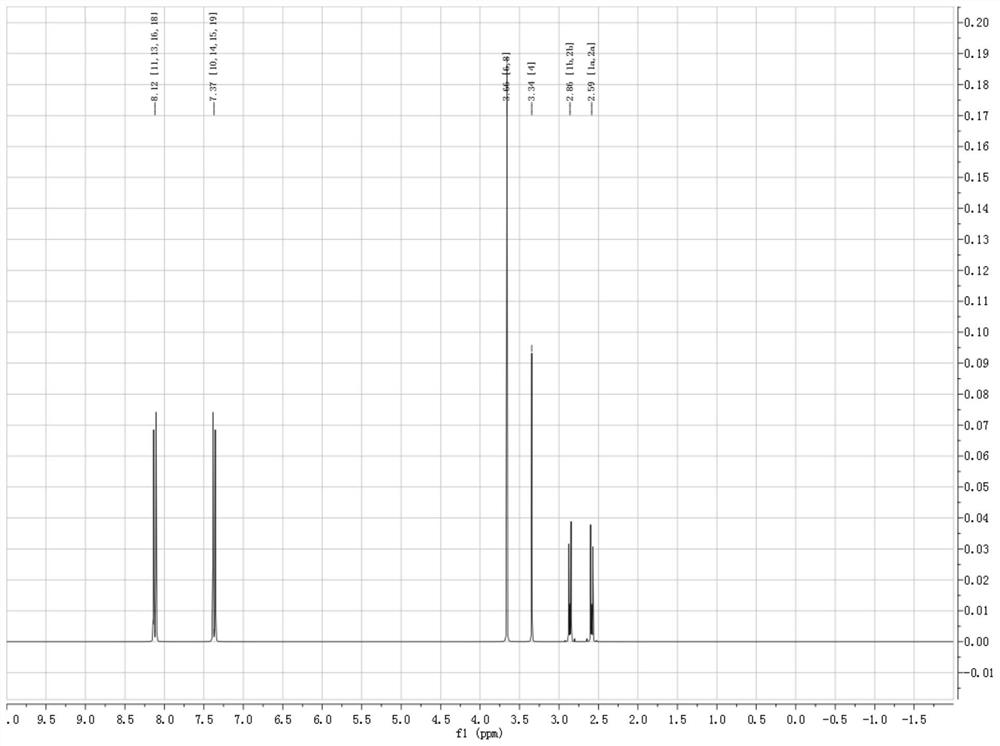
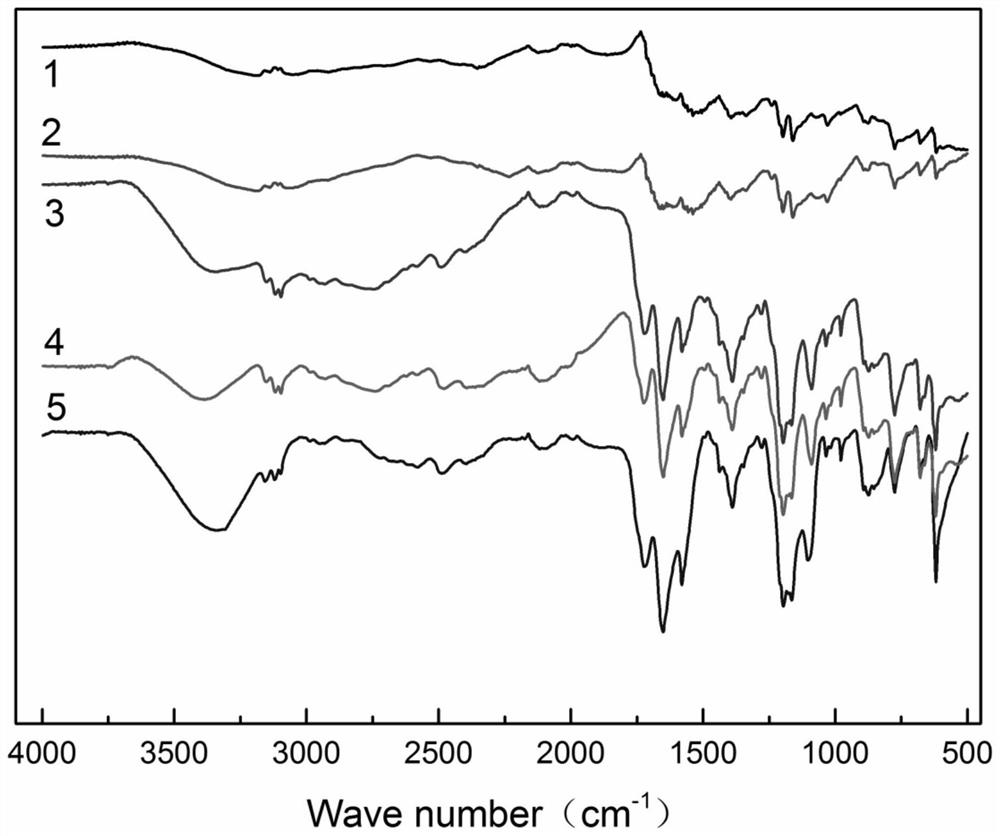
[0047]Step 3. Dissolve 1 mol of copper nitrate and 0.5 mol of intermediate Cl-II in 100 ml of deionized water, and sonicate. After the dissolution is completed, put it into a reaction kettle, and react at 61 °C for 3.5 h. After standing, suction filtration, and drying That is, the metal-based ionic liquid catalyst Cu-MZF-Cl is obtained, and the infrared spectrum is as follows image 3...
Embodiment 2
[0049] Step 1. Mix 0.5 mol of imidazole with 0.5 mol of dibromomethane, react at 80° C. for 20 h, wash with ethyl acetate, spin-evaporate, and vacuum dry to obtain intermediate Br-I, respectively.
[0050] Step 2. N 2 Under the protection, 0.75mol of benzoic acid was added to the reactor containing the above-mentioned 0.5mol of intermediate Br-I, stirred and dissolved, condensed and refluxed, and the reaction time was controlled at 11h. After the reaction, washed with acetonitrile, rotary evaporated, and vacuum dried Afterwards, different kinds of intermediates Br-II were obtained respectively.
[0051] Step 3. Dissolve 1 mol of ferric nitrate and 1 mol of intermediate Cl-II in 100 ml of deionized water, and sonicate. After the dissolution is completed, put it into a reaction kettle, and react at 67 ° C for 5.0 h. The metal-based ionic liquid catalyst Fe-MZF-Br was obtained, and the infrared spectrum is as follows image 3 shown.
Embodiment 3
[0053] Step 1. Mix 0.5 mol of imidazole with 0.5 mol of diiodomethane, react at 90° C. for 18 h, wash with ethyl acetate, spin-evaporate, and vacuum dry to obtain intermediates I-I, respectively.
[0054] Step 2. N 2 Under the protection, the 0.3mol benzoic acid added to the reactor equipped with the above-mentioned 0.5mol intermediate I-I was stirred and dissolved, condensed and refluxed, and the reaction time was controlled at 12h. Different kinds of intermediates I-II are obtained.
[0055] Step 3. Dissolve 1 mol of ferric nitrate and 1.5 mol of intermediate Cl-II in 100 ml of deionized water, and sonicate. After the dissolution is completed, put it into a reaction kettle, and react at 63 ° C for 4.0 h. After standing, suction filtration, and drying That is, the metal-based ionic liquid catalyst Mg-MZF-I is obtained, and the infrared spectrum is as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com