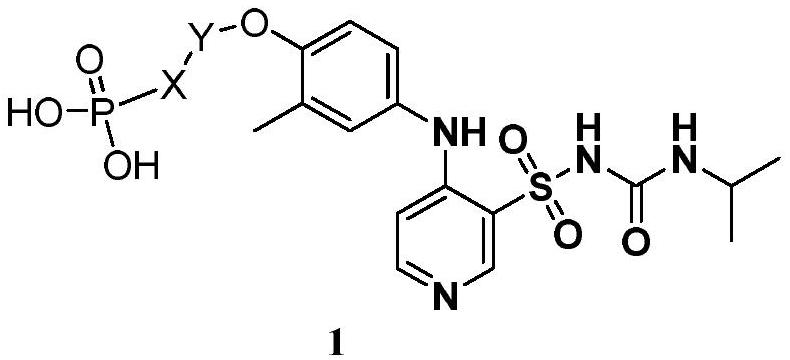
Application of pyridine sulfonamide phosphate compound in preparation of anti-encephaledema medicine
A technology of pyridine sulfonamide phosphate and compound, which is applied to pyridine sulfonamide phosphate compounds or pharmaceutical compositions containing them, in the field of preparation of anti-cerebral edema drugs, and achieves the effect of reducing intracranial pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
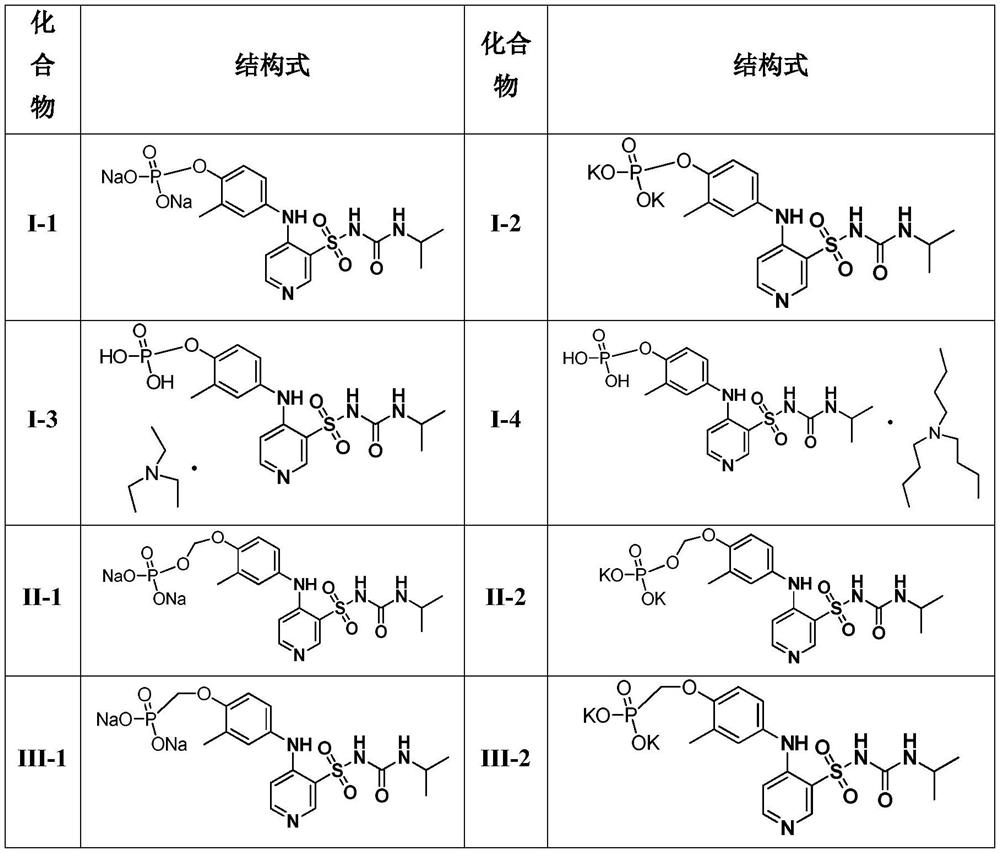
Embodiment 1
[0035] Activity study of the compound of Example 1 on nitroglycerin-induced intracranial hypertension model in experimental rabbits
[0036] Male experimental rabbits were divided into groups according to their body weight: normal control group, model control group, mannitol group (1000mg / kg), torasemide group (10mg / kg), and test drug group (10mg / kg). 4. Animals in each group were anesthetized and fixed. The normal control group was intravenously injected with 0.9% sodium chloride injection 5 mL / kg, and the other groups were given continuous intravenous infusion of nitroglycerin (0.04 mg / kg / min) for 10 minutes to establish models, except for the model control group. In addition to no drug treatment, the other groups were given the corresponding drugs by intravenous injection. The mannitol injection was an aqueous solution with a concentration of 20% (weight percent), and both torasemide and the test drug were administered with normal saline (ie, 0.9%). Sodium chloride injecti...
Embodiment 2
[0039] Activity study of the compound of Example 2 on collagenase IV-induced experimental rabbit cerebral hemorrhage model
[0040] The experimental rabbits were randomly divided into groups according to their gender and weight, which were the normal control group, the model control group, the mannitol injection group, the test drug group, and the combined administration group. The normal control group was intravenously injected with 5 mL / kg of 0.9% sodium chloride injection, and the animals in the other groups were injected subdurally with 1 μL of 0.9% sodium chloride injection containing collagenase IV 0.5 μg / μL for modeling, except for the model control group. In addition to no drug treatment, the other groups were given the corresponding drugs by intravenous injection. The mannitol injection was an aqueous solution with a concentration of 20% (100% by weight), and the test drugs were all administered with normal saline (ie, 0.9% sodium chloride injection). ) is made into the...
Embodiment 3
[0045] The inhibitory activity of the compound of embodiment 3 on Na+, K+, Cl- pump
[0046] Detection of furosemide, torasemide, and serial concentrations of test drugs on porcine kidney Na + -K + -2Cl - Activity in a pump transport model. The concentration of rubidium ions in each group of buffer solutions was detected by atomic absorption spectrometry, and the concentration of each test substance / reference substance on Na was calculated according to the original concentration of RbCl added. + -K + -2Cl - Pump activity, at half effective concentration (EC 50 ) indicates activity.
[0047] The results show that the tested drugs have different degrees of inhibitory activity on Na+-K+-2Cl- pump activity, and have a dose-response relationship, EC 50 The data are shown in Table 3, and the effective rates of compounds I-1, II-1, and III-1 at different concentrations are shown in Table 4.
[0048] The activity of table 3 compounds on Na+-K+-2Cl- pump
[0049]
[0050] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com