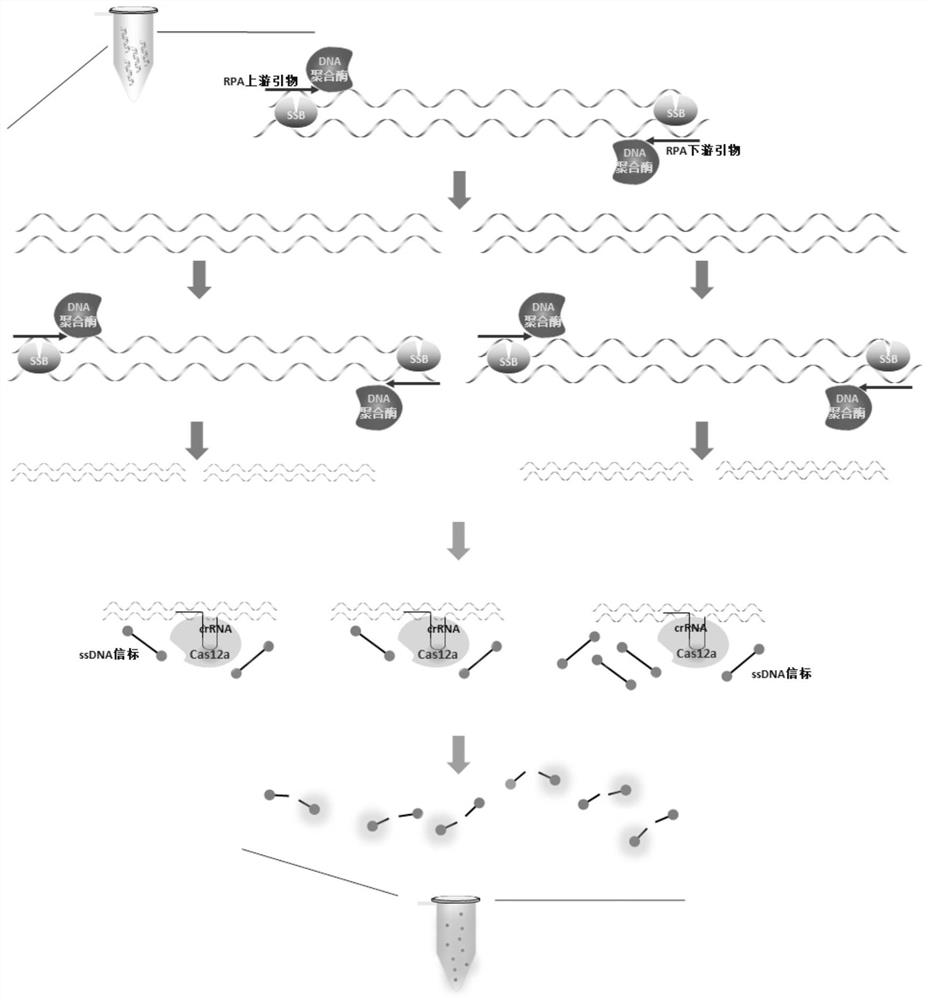
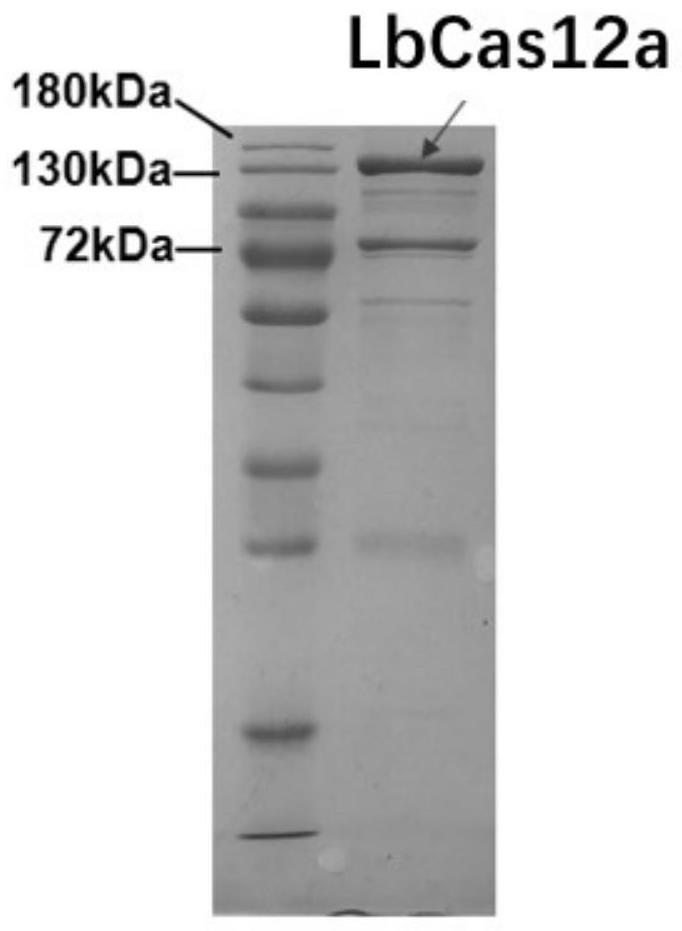
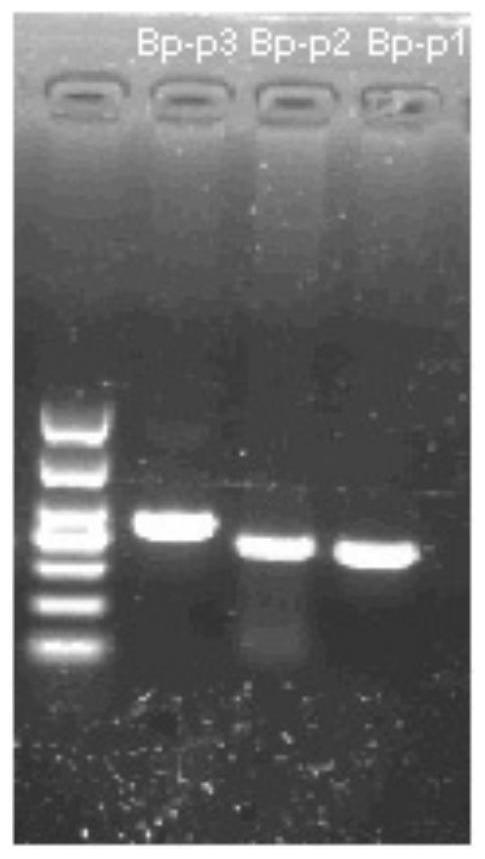
RPA-LbCas12a system-based composition for visual detection of nasal-like carbuncle and application of RPA-LbCas12a system-based composition
A composition, genome technology, applied in the direction of DNA/RNA fragments, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problems of wrong treatment, aerosol pollution, background interference of people, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1. Establishment of Burkholderia melioidosis RPA-LbCas12a detection system
[0043] 1. Selection of target sequences
[0044] Through analysis, we selected the nucleotide sequence of 2381740-2382182 bp on chromosome 1 of Burkholderia melioidans BPC006 strain (GenBank: CP003781.1) as the target sequence for Burkholderia melioidans gene detection, such as SEQ ID NO: 10.
[0045] Target sequence for Burkholderia melioidosis gene detection (443bp):
[0046] GGGACGCATACACTACCAGATTTGATAGTTTCGTCCTTTCAAAATCTAGACTCTAATAAACTCGCACACTTTTCCCATCACATCGATGGCGATTAACCAATAAATCCAGTGGAGTTAAAAATGGGCAAAGCGAATACCATCGAGCTCACAAACAACACATCATTTACTCTCGTCCTGCATACGATATACGCCAACACGGGCAATTGGTCCGGCGATTATCCGCCGGCCTATTTACGGCCGAACGATACGCTTATTTTTACGAGTACGCTTGATGGAAAAGGAGATCTAAACGGCTCAGCCCGTTTCGACATCCTTGATACAGCGGTCAAGAGATGTCCGGACGCGACCTACGTACAGCTCAACTGGGACAATCCCGTCGGAGCGGACAATGGGGGATCCTCGTCCGTAGTCGGCGCCACAGCACAGTTCTTCAACGTAAGTGG(SEQID NO:10)。
[0047] 2. Design of RPA primers and crRNA
[0048] For ...
Embodiment 2
[0101] Example 2. Evaluation of sensitivity and specificity of Burkholderia melioidosis RPA-LbCas12a detection system
[0102] Normal blood genomic DNA was extracted from human normal blood samples using Quick DNA / RNA Pathogen miniprep Kit (ZYMO RESEARCH, R1042) for the following sensitivity and specificity evaluation experiments. Human normal blood samples were provided by the Laboratory of Clinical Microbiology and Immunology, Department of Pharmacy and Laboratory Medicine, Army Medical University.
[0103] 1. Sensitivity evaluation
[0104] The genomic DNA of Burkholderia pseudomallei BPC006 strain quantified by real-time fluorescent quantitative PCR was added to the normal blood genomic DNA solution to obtain 1.35 copies / μL, 2.7 copies / μL, 27 copies / μL, 270 copies / μL, 2700 copies / μL copies / μL, 27,000 copies / μL, and 270,000 copies / μL of Burkholderia melioidans nucleic acid samples. Take 5 μL of Burkholderia melioidi nucleic acid samples of various concentrations as templa...
Embodiment 3
[0109] Example 3. ROC curve verification of Burkholderia melioidosis RPA-LbCas12a detection system
[0110] The normal blood samples used in this experiment were human normal blood samples, which were provided by the Laboratory of Clinical Microbiology and Immunology, Department of Pharmacy and Laboratory Medicine, Army Medical University. The Burkholderia pseudomallei bacteria solution used in this experiment was the Burkholderia pseudomallei BPC006 strain.
[0111] First prepare the following samples:
[0112] Sample 1: Take 20 normal blood samples and extract genomic DNA respectively;
[0113] Sample 2: Take 20 normal blood samples, add a certain amount of Burkholderia pseudomallei bacteria solution respectively, and then extract genomic DNA respectively; the number of copies of the genome DNA of Melioidosis in the extracted genomic DNA is about 1-2.7 copies / μL;
[0114] Sample 3: Take 20 normal blood samples, add a certain amount of Burkholderia pseudomallei bacteria s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com