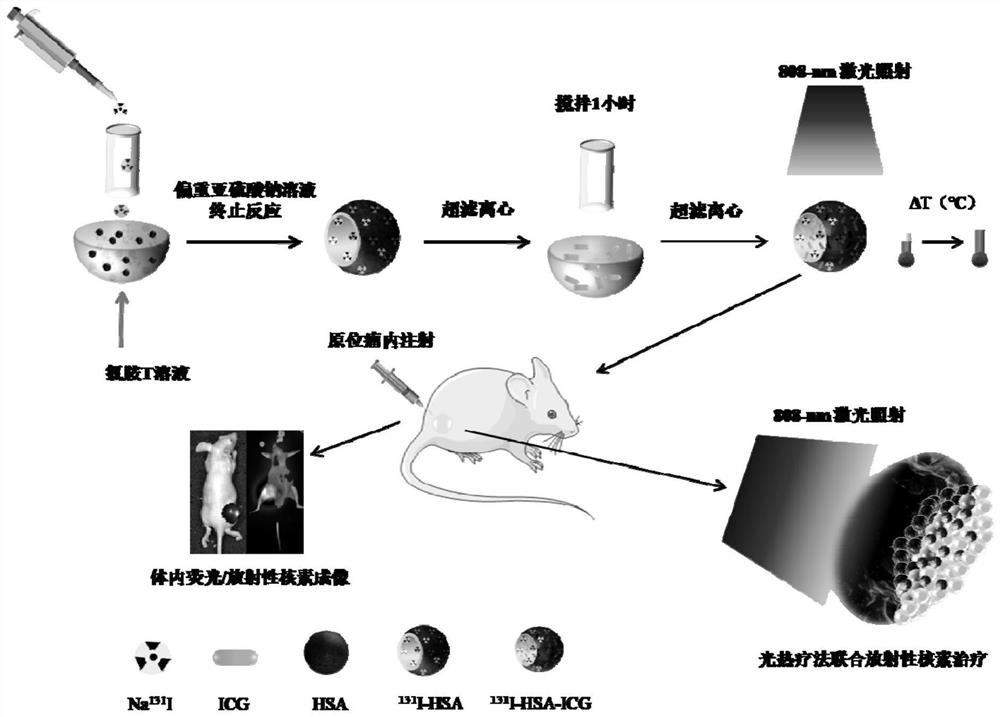
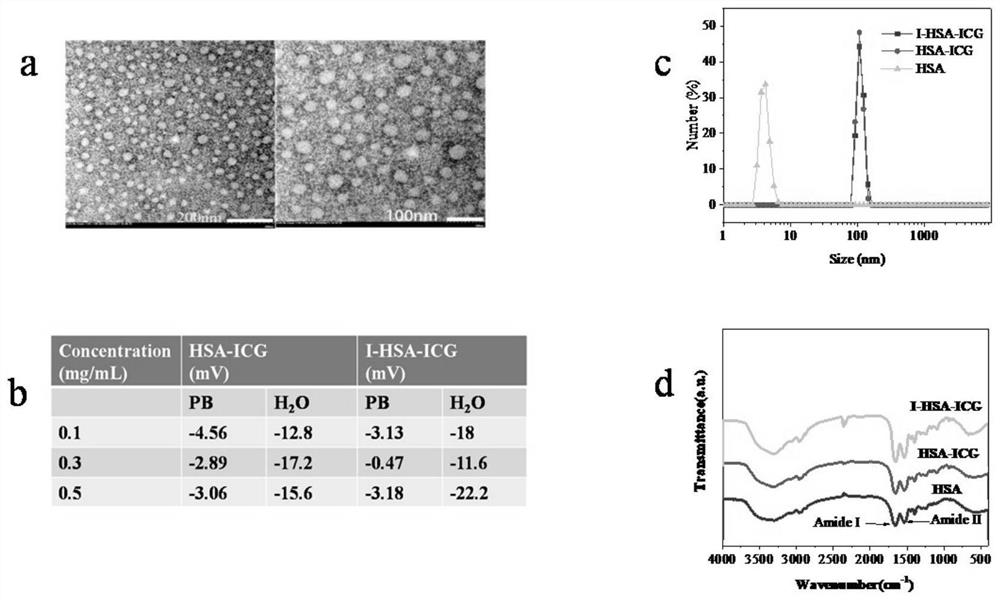
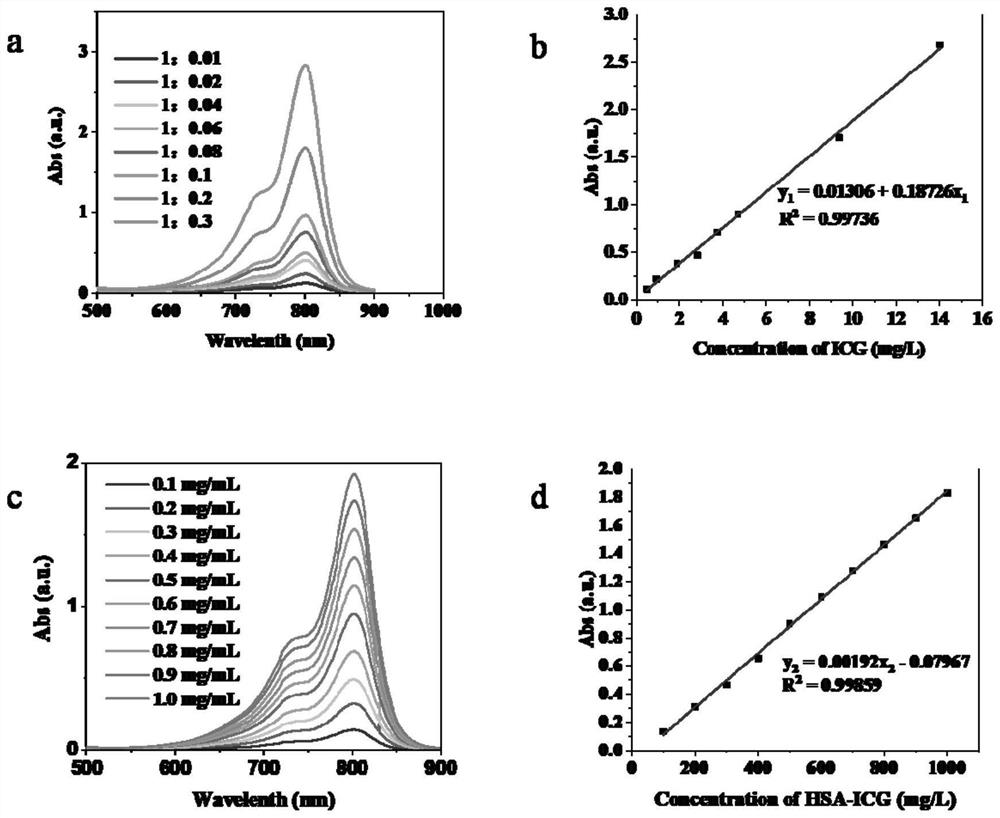
< 131 > I-HSA-ICG nanoparticles as well as preparation method and application thereof
A technology of 131I-HSA-ICG and nanoparticles, applied in the field of 131I-HSA-ICG nanoparticles and its preparation, to achieve the effects of high water solubility, high fluorescence efficiency and stability, and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0040] Preparation of HSA-ICG Nanoparticles
[0041] According to the molar ratio of HSA:ICG=1:1, weigh 20 mg of HSA and dissolve it in 4 mL of H 2 O, corresponding to ICG 0.234mg dissolved in 1mL H 2 O, the reaction system is 5mL, 0.117mL of 2mg / mL ICG solution can be mixed with water to 1mL, stirred and reacted with 4mL HSA solution for 1h, ultrafiltration and centrifugation (molecular weight cut-off 30kDa, rotating speed 6000r / min, 10min, rinse 3 times), the ultrafiltration The solution was expanded to 5 mL, and placed in a petri dish for 30 min in a refrigerator at 4°C, 2 hours in a -20°C refrigerator, and overnight in a -80°C refrigerator. The purified HSA-ICG nanoparticles were freeze-dried by a freeze dryer, and stored in a refrigerator at 4°C away from light for subsequent experiments.
Embodiment 1
[0043] Preparation of I-HSA-ICG nanoparticles
[0044] (1) Prepare 0.01M PB buffer: weigh 7.16g Na 2 HPO 4 -12H 2 O and 3.12g NaH 2 PO 4 -2H 2 O was dissolved in 100ml aqueous solution, and 19mL 0.2mol / mL Na 2 HPO 4 -12H 2 O solution, 81 mL of 0.2 mol / mL Na 2 HPO 4 -12H 2 O solution, mix evenly (0.2M, pH=7.4), take 50mL, add water and dilute to 1000mL for later use.
[0045] (2) Weigh 20 mg of HSA, 5 mg of chloramine T, and 5 mg of sodium metabisulfite, respectively, and dissolve them in 1 mL of PB buffer, NaI 2H 2 O 930 mg dissolved in 1 mL of water. HSA, chloramine T, NaI 2H 2 The O solution was mixed, and after the reaction was shaken by a shaker for 1 min, sodium bisulfite solution (5 mg / mL, 1 mL) was added to terminate the reaction (reaction for 1 min). The solution after the above reaction was ultrafiltered (molecular weight cut-off 30kDa, rotational speed 6000r / min, 10min, rinsed 3 times), and then high-purity water was added to expand the volume to 4mL. ...
Embodiment 2
[0047] 131 Preparation of I-HSA-ICG nanoparticles
[0048] According to the needs of the experiment, put on a lead coat, and extract a certain unit count of radioactive Na in the fume hood 131 I solution (volume is less than 1mL, the count is greater than the target count by at least 1 unit), label HSA according to the chloramine T method and ultrafiltration centrifuge (molecular weight cut-off 30kDa, rotating speed 6000r / min, 10min, rinse 3 times), re-measure the filtrate radioactivity counts (labeling rate above 90%), and then the radioactivity 131 The I-HSA solution is reacted with the prepared ICG solution (the steps are the same as above), and the reacted solution is ultrafiltered and centrifuged to 250 μL for use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thermal efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com