Responsive slow-release type polydopamine-Zn-natural polyphenol coordination nano-drug and preparation method thereof
A technology of natural polyphenols and polydopamine, which is applied in the direction of nano-drugs, drug combinations, nano-technology, etc., can solve problems such as the stability of natural polyphenols, and achieve the effect of enhancing stability and reducing the rate of deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
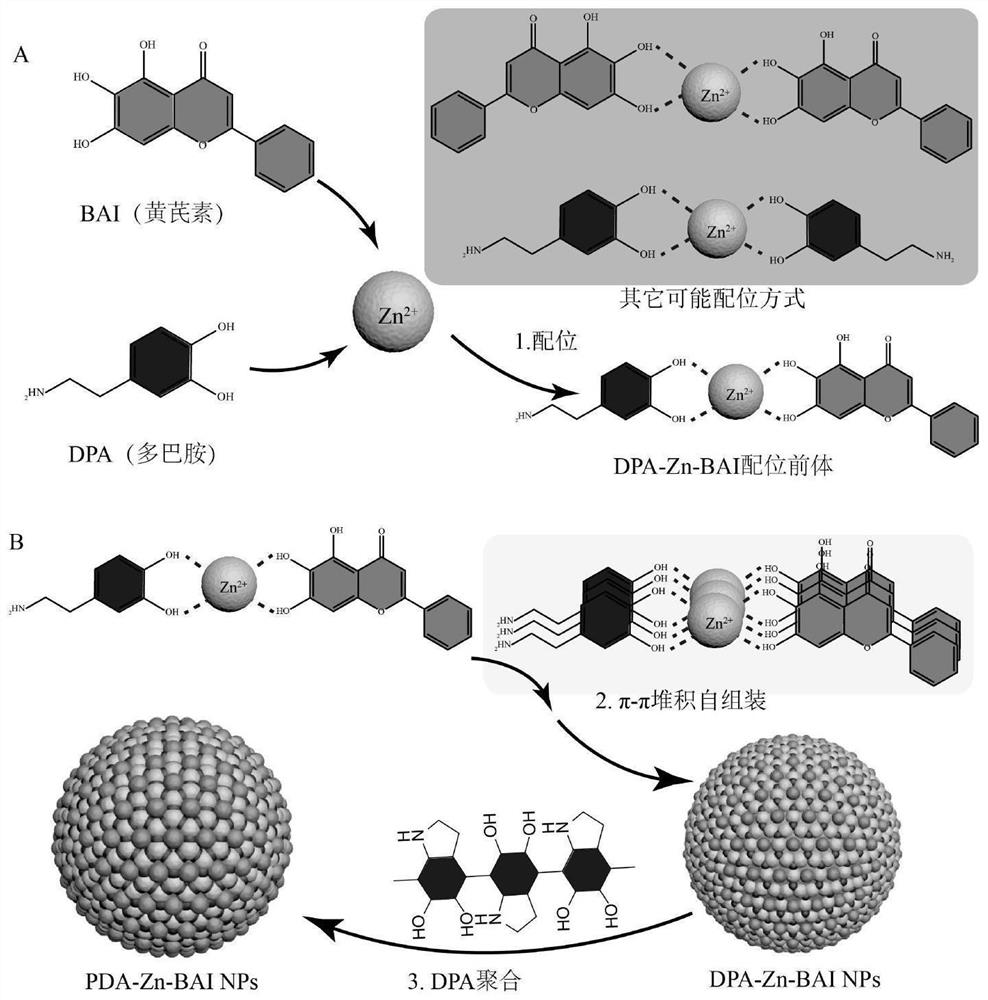
[0031] The invention provides a preparation method of a response slow-release polydopamine-Zn-natural polyphenol coordination nanomedicine, comprising the following steps:
[0032] (1) mixing natural polyphenol drug solution, dopamine solution and Zn ion solution to carry out coordination reaction to obtain coordination precursor solution;
[0033] (2) mixing the coordination precursor solution and the surfactant solution to carry out a self-assembly reaction to obtain self-assembled nanoparticles; the solvent of the surfactant solution is an alkaline buffer;
[0034] (3) mixing the self-assembled nanoparticles with an alkaline buffer to carry out a dopamine polymerization reaction to obtain a response slow-release polydopamine-Zn-natural polyphenol coordination nanomedicine.
[0035]In the present invention, the natural polyphenol drug solution, the dopamine solution and the Zn ion solution are mixed to carry out a coordination reaction to obtain a coordination precursor solu...
Embodiment 1
[0047] (1) Preparation of dopamine-Zn-astragalin (BAI) coordination precursor solution: First, astragalin, dopamine and ZnCl were mixed 2 Dissolved in DMSO, respectively, to obtain 5 mg / mL astragalin solution, 10 mg / mL dopamine solution and 10 mg / mL ZnCl 2 Solution; take 1mL of astragalus solution, mix 0.2mL of dopamine solution and place it on a magnetic stirrer and stir at 900rpm, then slowly add ZnCl dropwise to the mixture 2 The solution was 0.25 mL, and after the dropwise addition was completed, stirring was continued for 30 minutes at room temperature, and the dopamine-Zn-astragalin coordination precursor solution was obtained by the reaction. In the reaction, the input mass ratio of astragalin, dopamine and Zn ions was 5:2:1.19.
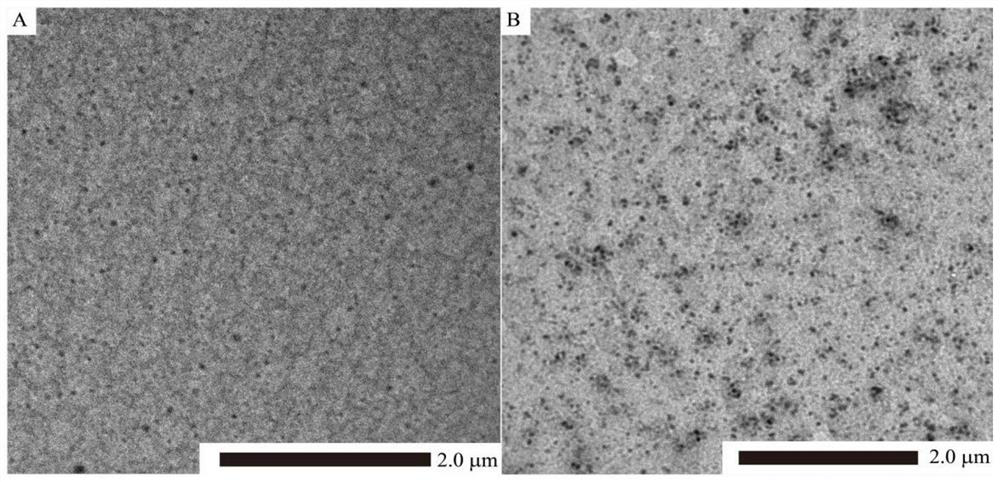
[0048] (2) Preparation of dopamine-Zn-astragalin coordination and π-π stacking self-assembled nanoparticles: Pluronic F127 was dissolved in 10 mM Tris-HCl buffer at a concentration of 1 mg / mL to form a surfactant solution . Take 10 mL of th...
Embodiment 2
[0075] (1) Preparation of dopamine-Zn-astragalin coordination precursor solution: firstly, astragalus and dopamine were dissolved in methanol to obtain 5 mg / mL astragalin solution and 10 mg / mL dopamine solution, and Zn(NO) 3 ) 2 Dissolved in DMSO to obtain 10 mg / mL of Zn(NO 3 ) 2 solution; take 1 mL of astragalus solution, mix 0.5 mL of dopamine solution and place it on a magnetic stirrer and stir at 900 rpm, then slowly add Zn(NO) dropwise to the mixture 3 ) 2 The solution was 0.2 mL, and after the dropwise addition was completed, stirring was continued for 60 minutes at room temperature, and the astragalin-Zn-dopamine coordination precursor solution was obtained by the reaction. In the system, the input mass ratio of astragalin, dopamine and Zn ions was 5:5:0.69.
[0076] (2) Preparation of dopamine-Zn-astragalin coordination and π-π stacking self-assembled nanoparticles: Pluronic F127 was dissolved in 5 mM Tris-HCl buffer at a concentration of 2 mg / mL to form a surfacta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com