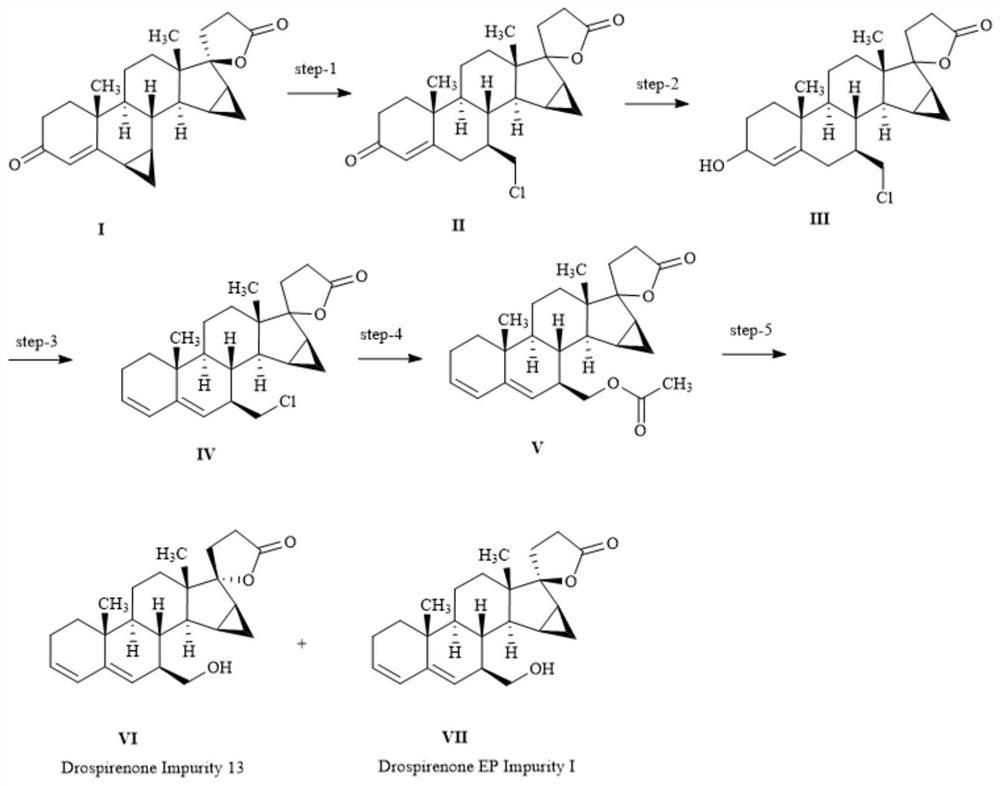
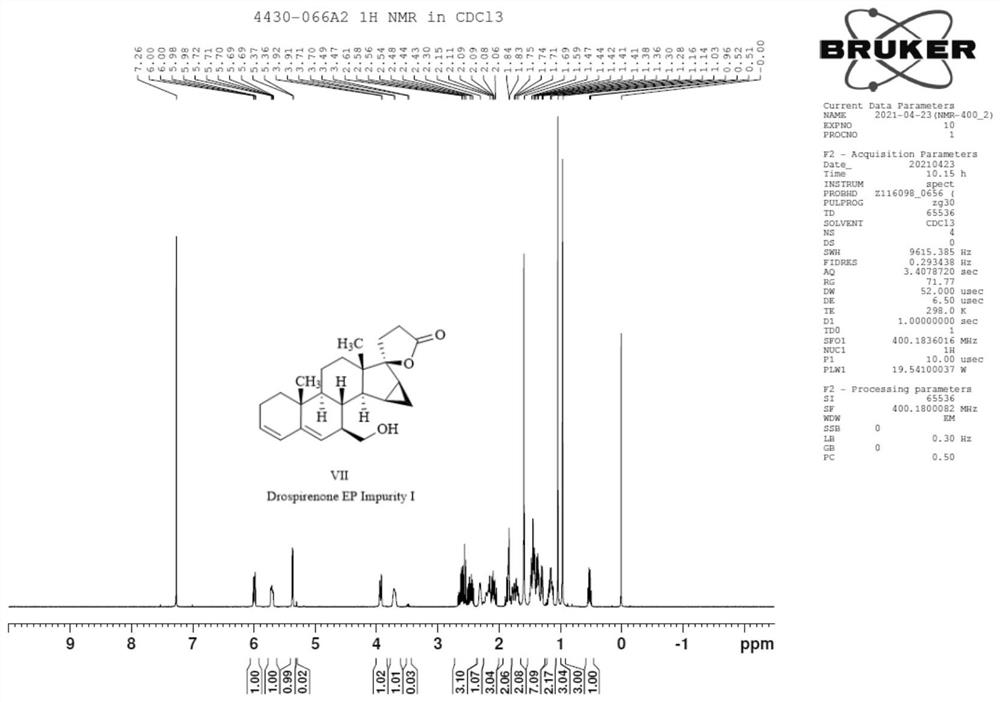
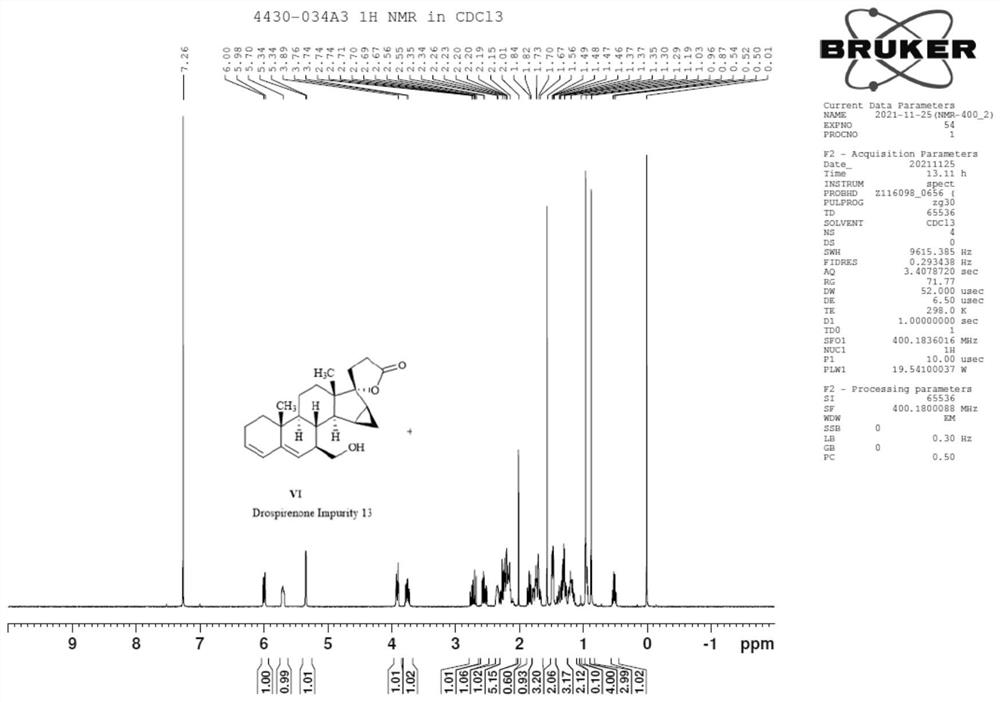
Preparation method of drospirenone impurity
A drospirenone and impurity technology, which is applied in the preparation of compounds and progesterone drospirenone impurities, can solve the problems of difficult removal and easy residue, and achieve the effects of convenient purification, high reaction efficiency and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation method of described drospirenone impurity, its preparation technological process is as follows figure 1 shown, including the following steps:
[0034] (1) Raw material 70g Drospirenone (I, the structure is as follows Figure 4 ) was dissolved in acetone, concentrated hydrochloric acid was added dropwise to the ice bath, and then the temperature was raised to 70 °C for 3 hours. TLC spot plate showed no starting material remaining. The reaction solution was cooled to room temperature, adjusted to pH 8 with 17.5 g of sodium carbonate dissolved in 250 ml of water, and then spin-dried. 0.5 L of water was added to the system, and extracted with dichloromethane (200 mL x 3). The organic phase was washed once with water, dried over anhydrous sodium sulfate, filtered, concentrated and recrystallized with ethyl acetate to obtain a solid, which was filtered and dried to obtain 58 g of intermediate II (mixture of IIa and IIb) with a yield of 75.36%.
[0035] MS ...
Embodiment 2
[0046] The preparation method of described drospirenone impurity, its preparation technological process is as follows figure 1 shown, including the following steps:
[0047] (1) 50 g of the raw material Drospirenone was dissolved in acetone, 19 ml of concentrated hydrochloric acid was added dropwise in an ice bath, and then the temperature was raised to 70° C. to react for 3 hours. TLC spot plate showed no starting material remaining. The reaction solution was cooled to room temperature, adjusted to pH 8 by dissolving 16.5 g of sodium carbonate in 220 ml of water, and then spin-dried. 0.35 liter of water was added to the system, and the mixture was extracted with dichloromethane (160 mL×3). The organic phase was washed once with water, dried over anhydrous sodium sulfate, filtered, concentrated and recrystallized with ethyl acetate to obtain a solid, which was filtered and dried to obtain 45.6 g of intermediate II (mixture of IIa and IIb). The structures of structural formu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com