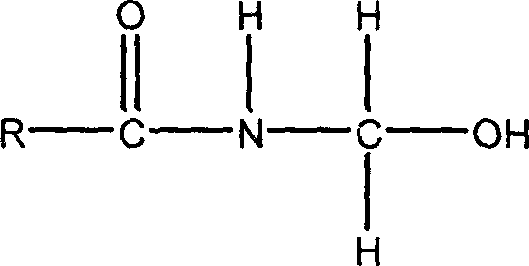
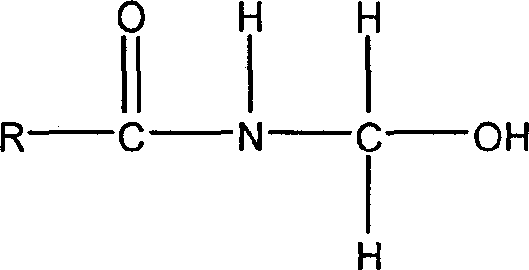
Synthetic method for amide derivatives
A technology of amide derivatives and synthesis methods, which is applied in the field of amide derivatives, can solve the problems of strong acid corrosiveness of catalysts, difficulty in separating and reusing unreacted raw materials, etc., and achieve the effect of easy operation and corrosion avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] The synthesis of embodiment 1.N-(4-hydroxyl-3-methoxy-phenylmethyl) acrylamide
[0010] Experimental procedure: (1) in 1000ml there-necked flask, add the guaiacol (o-methoxyphenol) of 50.8 grams (0.41mol), the N-methylol acrylamide of 40.4 grams (0.4mol), 200 grams of acid Treated 732-type cation exchange resin, then slowly add 400ml of absolute ethanol under stirring, and pass through N 2 (to prevent the hydroxyl group from being oxidized), the reaction was stirred at room temperature (about 30° C.) for 7-8 days.
[0011] (II) Filter the reaction mixture, wash the H-type cation exchange resin several times with absolute ethanol, and combine the washing solution and the mother liquor.
[0012] (III) The obtained liquid was evaporated to dryness under reduced pressure at 50°C, the residue was washed with ether, dried and then washed with water to obtain a crude product.
[0013] (IV) After dissolving the crude product with dilute lye, remove the insoluble matter by fil...
Embodiment 2
[0014] The synthesis of embodiment 2.N-(3,4-dihydroxy-benzyl) acrylamide
[0015] Experimental procedure: (1) in 1000ml there-necked flask, add the catechol of 44.0 grams (0.41mol), the N-methylolacrylamide of 40.4 grams (0.4mol), the 732 type cation after the acid treatment of 200 grams Exchange resin, then slowly add 400ml acetic acid under stirring, and pass into N 2 , the reaction was stirred at room temperature (about 30° C.) for 7-8 days.
[0016] (II) Filter the reaction mixture, wash the H-type cation exchange resin several times with acetic acid, and combine the washing solution and the mother liquor.
[0017] (III) The obtained liquid was evaporated to dryness under reduced pressure at 50°C, the residue was washed with ether, dried and then washed with water to obtain a crude product.
[0018] (IV) After dissolving the crude product with dilute lye, remove the insoluble matter by filtration, and slowly add dilute acid to adjust the pH to 2-3 to obtain a khaki-yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com