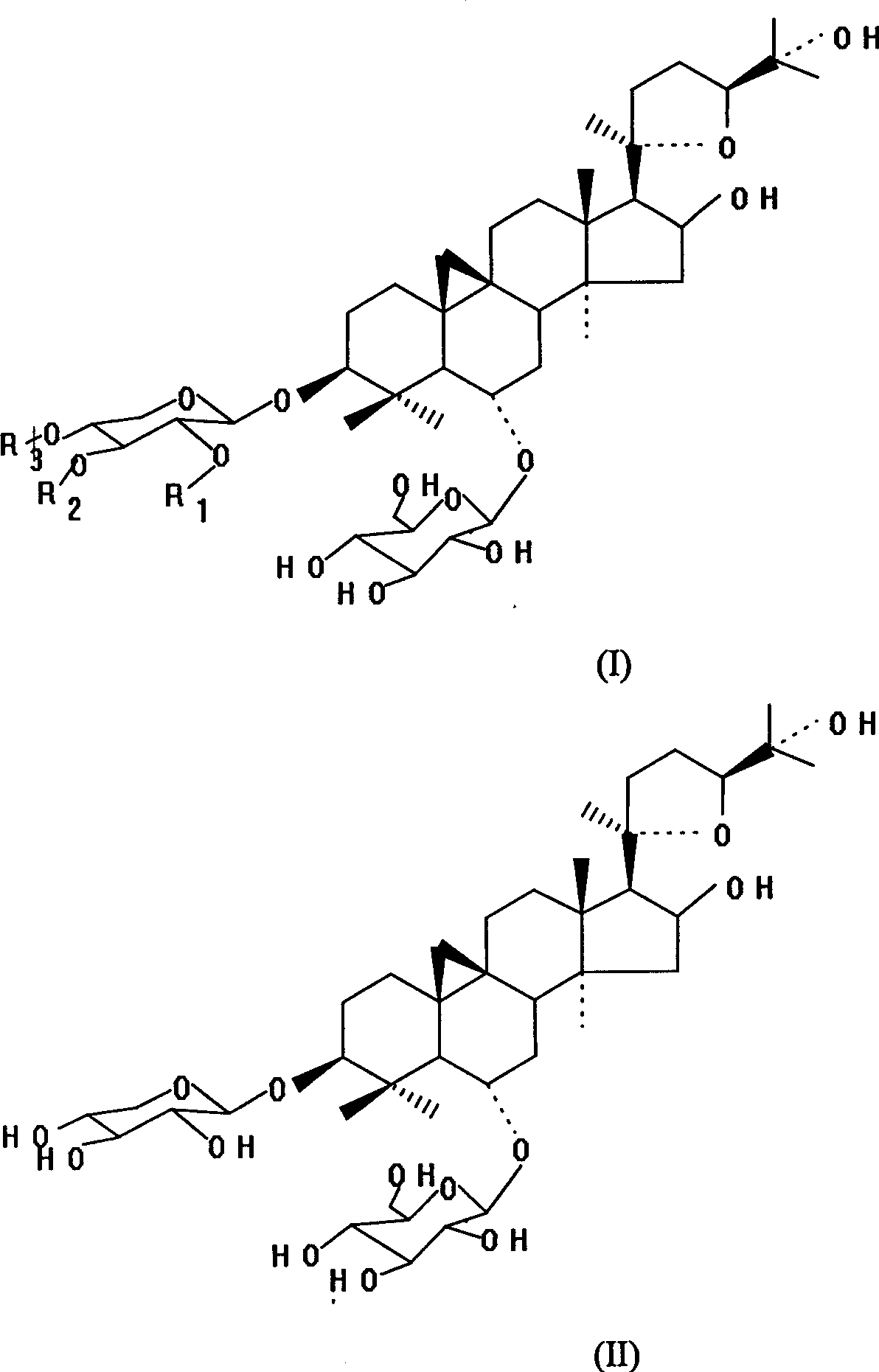
Astragalus root methyl-glycoside composition and preparation method
A technology of astragaloside IV and its composition, which is applied in the field of astragaloside IV composition and its preparation, can solve the problems of low astragaloside IV, high cost, and long cycle, and achieve the effects of good stability, clear ingredients, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take the dried raw material of Astragalus membranaceus and cut it into 1-3mm thin slices, put it into a hot reflux extraction tank, soak it with 10 times the amount of water for 1 hour, then heat and reflux for extraction for 4 hours, release the extract, filter, and collect the filtrate.
[0041] Take HPD-100 macroporous resin, which is 1 / 2 the amount of medicinal materials, and fill the column with water wet method, wash with ethanol until the eluent is not turbid after adding water, and then wash with water until no ethanol remains. Pass the above-mentioned filtrate through the macroporous resin column. After loading the column, rinse it with water, and then use 75% ethanol to elute at a rate of 1000 ml / min. Collect the eluate and detect it at any time. Does not contain total astragalosides (usually counted as astragaloside IV), and the eluate is concentrated to dryness at 70°C under reduced pressure to obtain crude brown-yellow total astragalosides.
[0042] Dissolv...
Embodiment 2
[0044] Take the dried Astragalus raw material and cut it into thin slices of 1-3 mm, put it into a hot reflux extraction tank, soak it with 15 times the amount of water for 1 hour, then heat it for reflux extraction for 4 hours, release the extract, filter, and collect the filtrate.
[0045]Take HPD-300 macroporous resin with 1 / 2 the amount of medicinal materials, wet-pack the column with ethanol, wash with ethanol until the eluent is not turbid after adding water, and then wash with water until no ethanol remains. Pass the above-mentioned filtrate through the macroporous resin column. After the column is loaded, rinse it with water, and then use 80% ethanol to elute at a rate of 1200 ml / min. Collect the eluate, detect it at any time, and add it to the eluate Does not contain astragaloside components (usually counted as astragaloside IV), and the eluate is concentrated to dryness at 65°C under reduced pressure to obtain crude brown-yellow total astragalosides.
[0046] Dissolv...
Embodiment 3
[0048] Take the dried Astragalus raw material and cut it into thin slices of 1-3 mm, put it into a hot reflux extraction tank, soak it with 15 times the amount of water for 1 hour, then heat it for reflux extraction for 4 hours, release the extract, filter, and collect the filtrate.
[0049] Take 2 / 3 of the HPD-400 macroporous resin of the medicinal material, wet-pack the column with ethanol, wash with ethanol until the eluent is not turbid after adding water, and then wash with water until no ethanol remains. Pass the above-mentioned filtrate through a macroporous resin column. After the column is loaded, rinse it with water, and then use 90% ethanol to elute at a rate of 1500 ml / min. Collect the eluate, detect it at any time, and add it to the eluate Does not contain astragaloside components (usually counted as astragaloside IV), and the eluate is concentrated to dryness under reduced pressure at 75°C to obtain crude brown-yellow total astragalosides.
[0050] Dissolve the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com