Process for preparing phloroglucinol
A technology of phloroglucinol and aniline, applied in the field of preparation of phloroglucinol, can solve the problems of inconvenient operation, harsh reaction conditions, poor color and luster, and achieves improved stability, reduced waste liquid volume, and good color luster. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
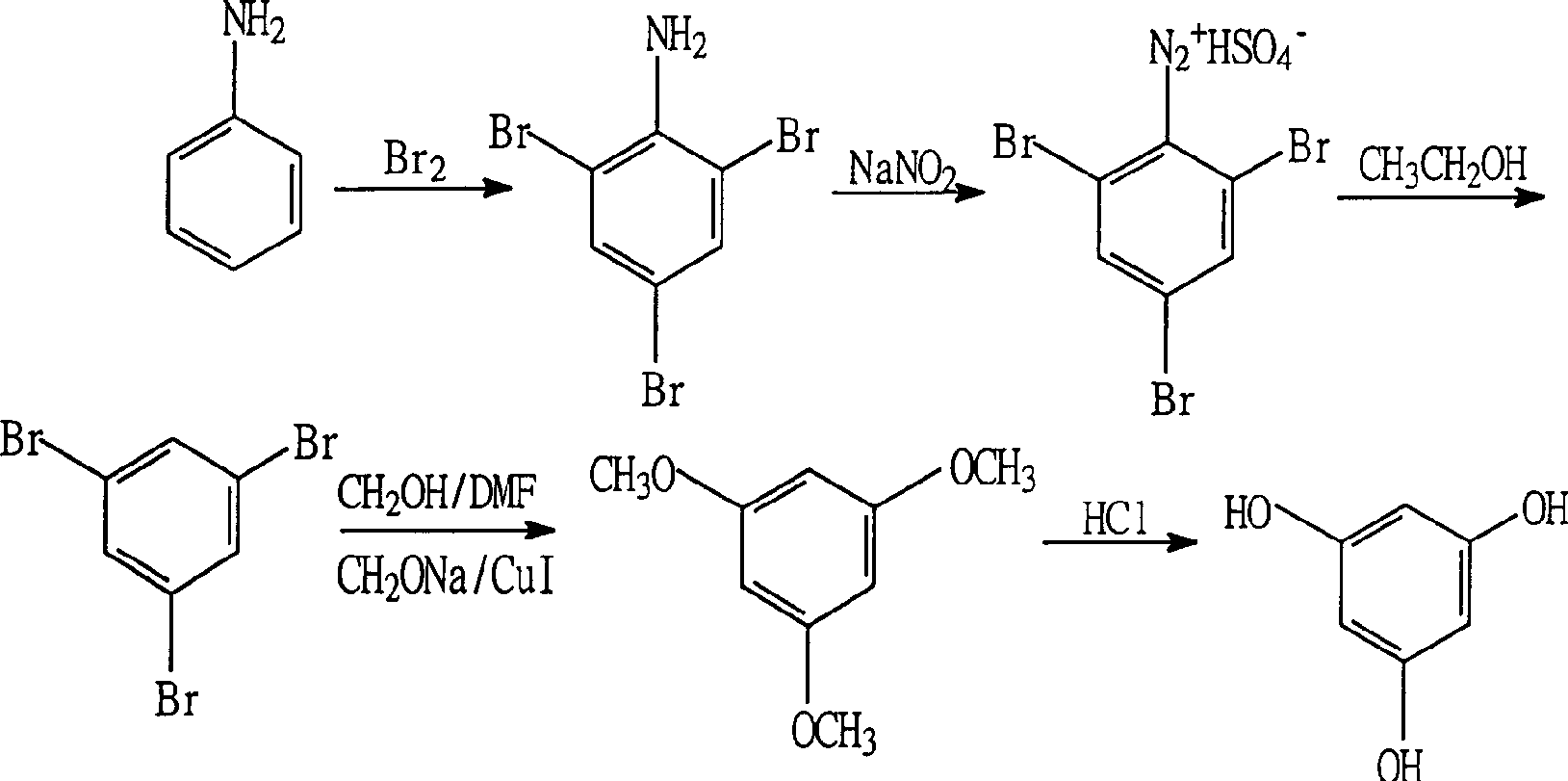
[0015] Embodiment 1 (2,4, the preparation of 6-tribromoaniline)
[0016] Add 100 ml of 10% hydrobromic acid aqueous solution and 10 g (0.1075 mol) of aniline to a 1000 ml three-neck flask equipped with a thermometer, a dropping funnel and a stirring device, and cool to below 10° C. in an ice-water bath. Dissolve 54.2g (0.3387mol) of liquid bromine in 600ml of 10% hydrobromic acid to prepare dilute bromine water. Add the prepared bromine water into the aniline solution within 30 minutes through the dropping funnel, and keep the temperature below 10°C. After the addition, the reaction was stirred for 10 minutes to end the reaction. The reaction solution was filtered under reduced pressure, the filter cake was washed with water until neutral, and the resulting solid was dried at 75°C to obtain 2,4,6-tribromoaniline as a white solid weighing 34.7g with a content of more than 99% .
Embodiment 2
[0017] Embodiment 2 (1,3, the preparation of 5-tribromobenzene)
[0018] Add 95% industrial ethanol 250ml and 100.0g dry 2,4,6-tribromoaniline solid and 0.5g copper sulfate pentahydrate in the 500ml four-neck flask with thermometer, dropping funnel, reflux device and stirring device, heat up to 50°C, stirring and dissolving for 15 minutes. Add 59.4g of concentrated sulfuric acid into the ethanol solution through the dropping funnel within 30 minutes, and the temperature is controlled at about 50°C. After the addition, keep at about 50°C and continue to stir for 15 minutes. 25.0g solid NaNO 2 Dissolved in water, dubbed a saturated aqueous solution. Prepared NaNO 2 The aqueous solution was added dropwise to the ethanol solution at 50°C within 2.0 hours through the dropping funnel. After the addition, continue to maintain 50°C, stir and react for 1.0 hour, then raise the temperature to reflux state, keep the reflux reaction for 2.0 hours, and end the reaction. The reaction ...
Embodiment 3
[0019] Embodiment 3 (1,3, the preparation of 5-tribromobenzene)
[0020] According to the method of Example 2, the feeding amount of copper sulfate pentahydrate was 2.0 g, and 91.2 g of 1,3,5-tribromobenzene was obtained after the reaction, with a content of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com