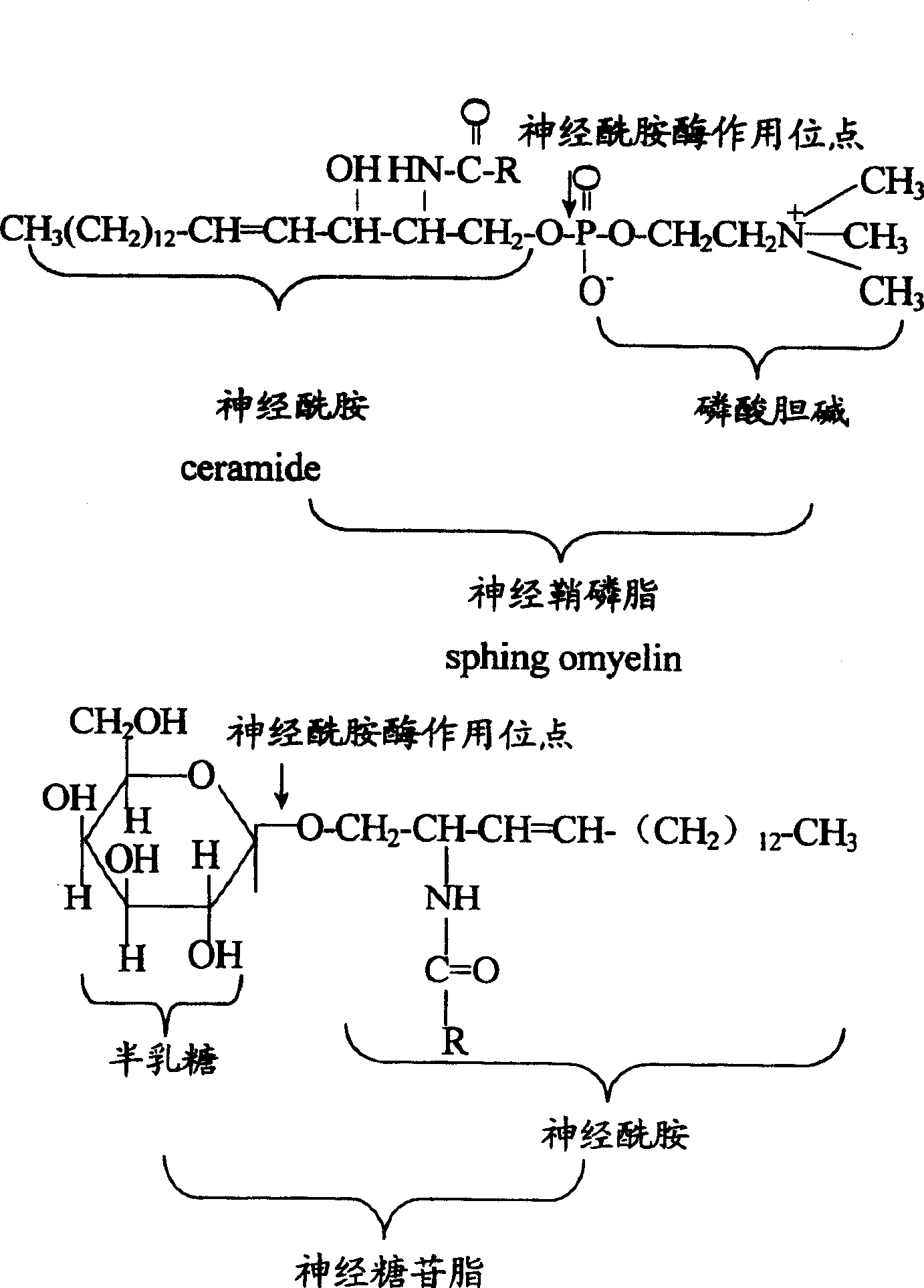
Application of Actinomucor elegans in use for preparing ceramide
A technology of Yazhi mucormyces radiata and ceramide, applied in the biological field, can solve problems such as threats to the health and life safety of the people, impacts on feed and breeding products export earnings, and excessive residues of prohibited drugs, etc., to reduce feeding costs, low cost, The effect of improving the absorption and utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 ceramide
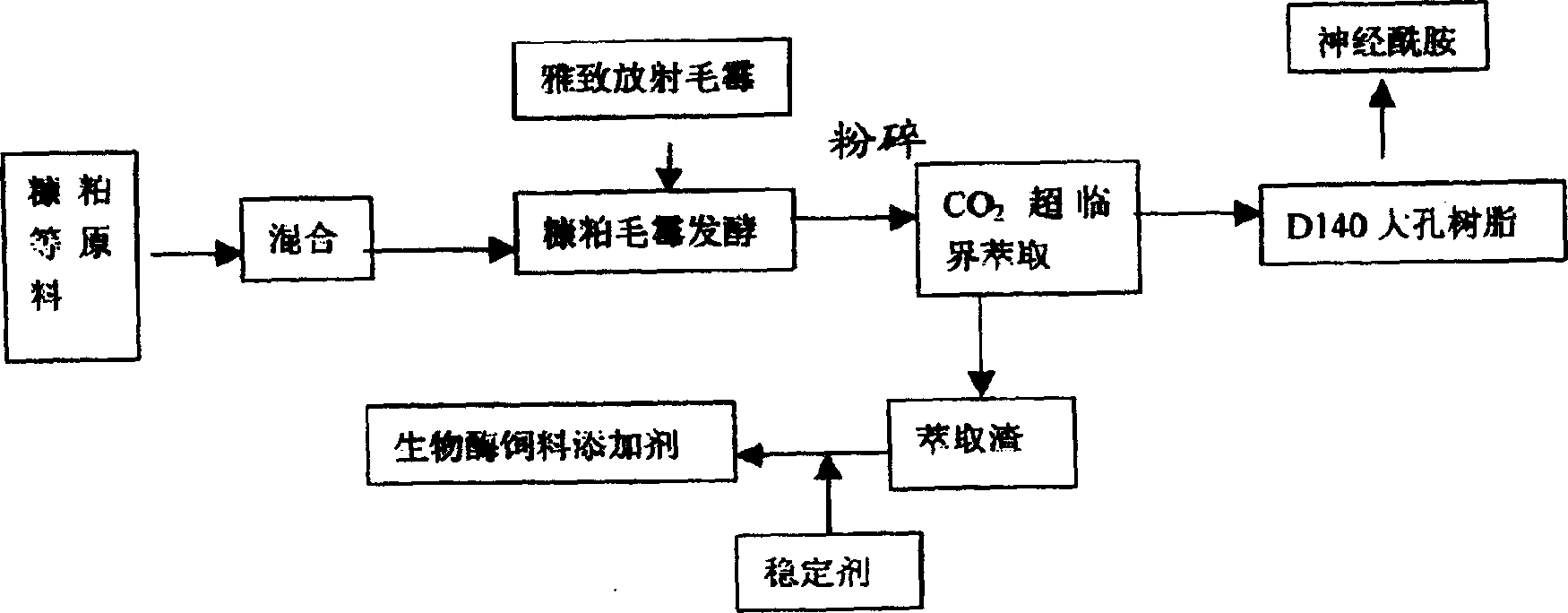
[0042] The invention provides a method for preparing ceramide, which is prepared by extracting rice bran as a raw material through bio-fermentation technology, and includes the following steps:
[0043] a. Select strains: select Actinomucor elegans from the General Microorganism Center of China Committee for Microbial Culture Collection: AS3.2778; the amount of strains is 4-12g;
[0044] b. Strain fermentation: carry out solid fermentation of selected strains, and the solid fermentation conditions are:
[0045] Solid medium: rice bran 98.43%, yeast extract 0.7%, KH 2 PO 4 0.1%, K 2 HPO 4 0.1%, MgSO 4 0.05%, CaCl 2 0.05%, ZnSO 4 0.02%, rapeseed oil 0.55%;
[0046] Cultivation temperature: 15℃~35℃;
[0047] Culture time: 24~84h;
[0048] Medium pH value: 7.0~8.0;
[0049] Medium water content: 40% to 70%;
[0050] Medium inoculum volume: 4% to 12%.
[0051] The culture medium adopts variable temperature culture,...
Embodiment 2
[0062] Qualitative and quantitative analysis of embodiment 2 product ceramide
[0063] 1. The ceramide prepared by the method of the present invention is detected by the Sichuan Provincial Center for Disease Control and Prevention, and the inspection basis is: GB / T5009.11-1996 and GB4789-94. The hygienic indicators are as follows:
[0064] Product heavy metal residue: see Table 1
[0065] Item Unit Measurement result
[0066] Product microbial indicators: see Table 2
[0067] Item Unit Measurement result
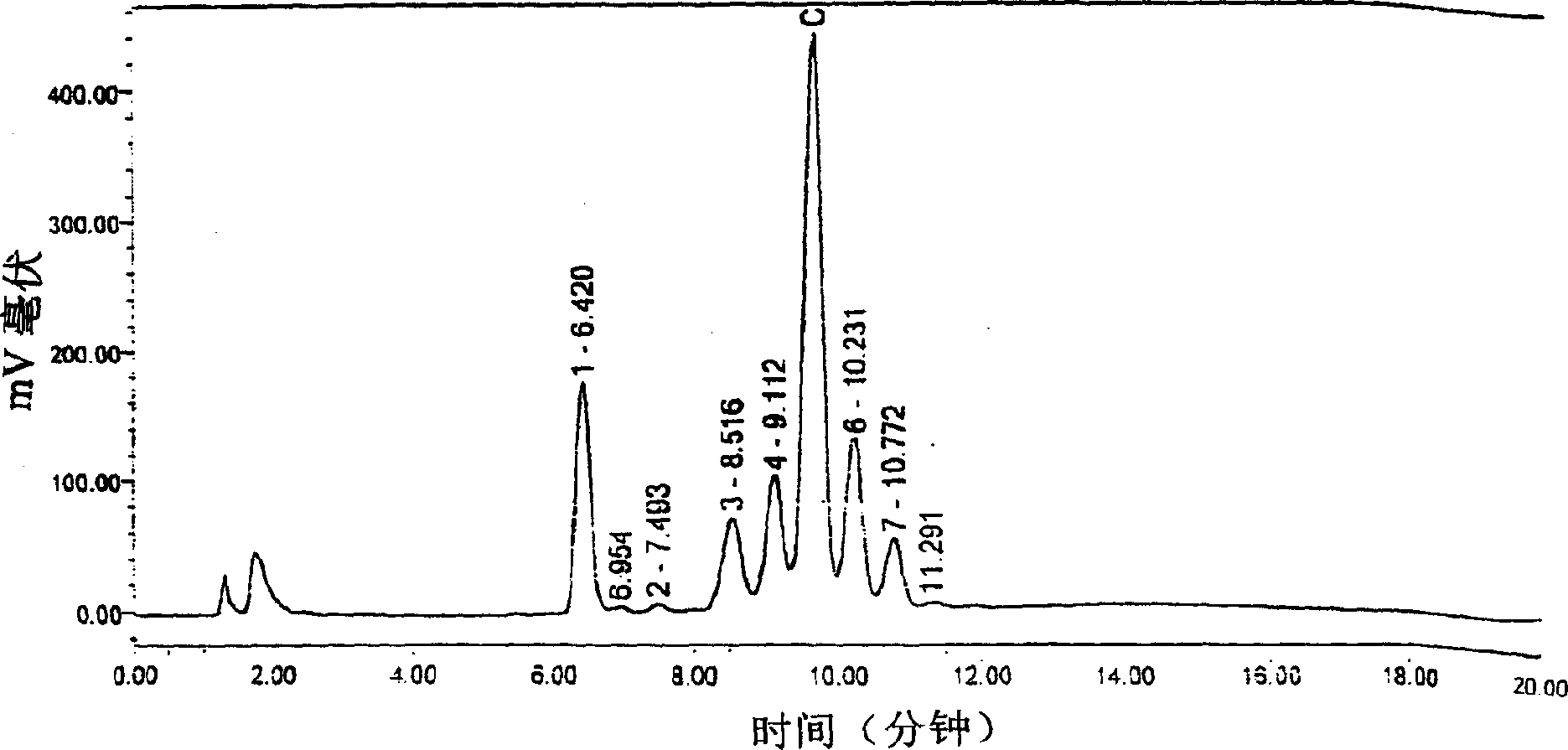
[0068] 2. The ceramide (ceramide) prepared by the method of the present invention is detected by the Waters liquid chromatograph at the Chengdu Branch Analysis and Testing Center of the Chinese Academy of Sciences, wherein the ceramide reference substance is a product of Sigma Company, and by comparing with the reference substance, the ceramide content is 101.8 %, purity and HPLC spectrum are basically consistent with the standard product of Sigma Compan...
Embodiment 3
[0078] Embodiment 3 Preparation of by-product enzyme-type feed additive of the method of the present invention
[0079] The remaining product 98.2~99.6% that embodiment 1 separates gained is added following stabilizer: calcium lactate, sorbitol make enzyme type feed additive stabilizer, the consumption scope of calcium lactate 0.2~0.8%, optimum consumption 0.5%; The dosage range is 0.2-1.0%, and the optimal dosage is 0.6%.
[0080] Preparation method: take 98.9 kg of solid fermentation raw materials before fermentation, calculate the respective addition amounts of calcium lactate and sorbitol, and add 10 times the amount of boiling water to dissolve it based on the total amount of 0.5 kg of calcium lactate and 0.6 kg of sorbitol Finally, it is sprayed on the residue after extracting ceramide, stirred evenly, air-dried at 30°C to 50°C, pulverized, packaged, and tested to become the finished product.
[0081] The beneficial effect of the by-product enzyme-type feed additive pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com