Productive method of recombinant human liver regenerated enhanced factor
A technology for enhancing factors and production methods, which can be used in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as lack of liver specificity, and achieve the effect of improving survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Production method of recombinant full-length human enhancer of liver regeneration factor
[0032] One, the technical points of this embodiment:
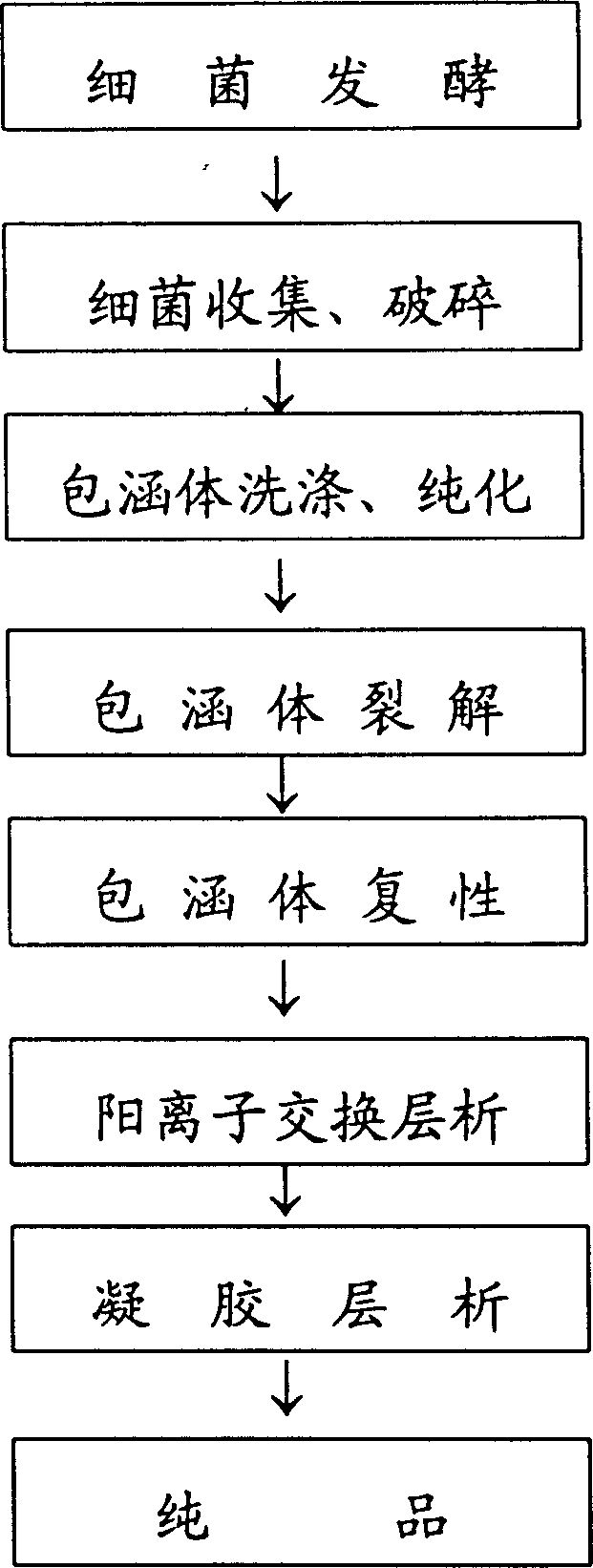
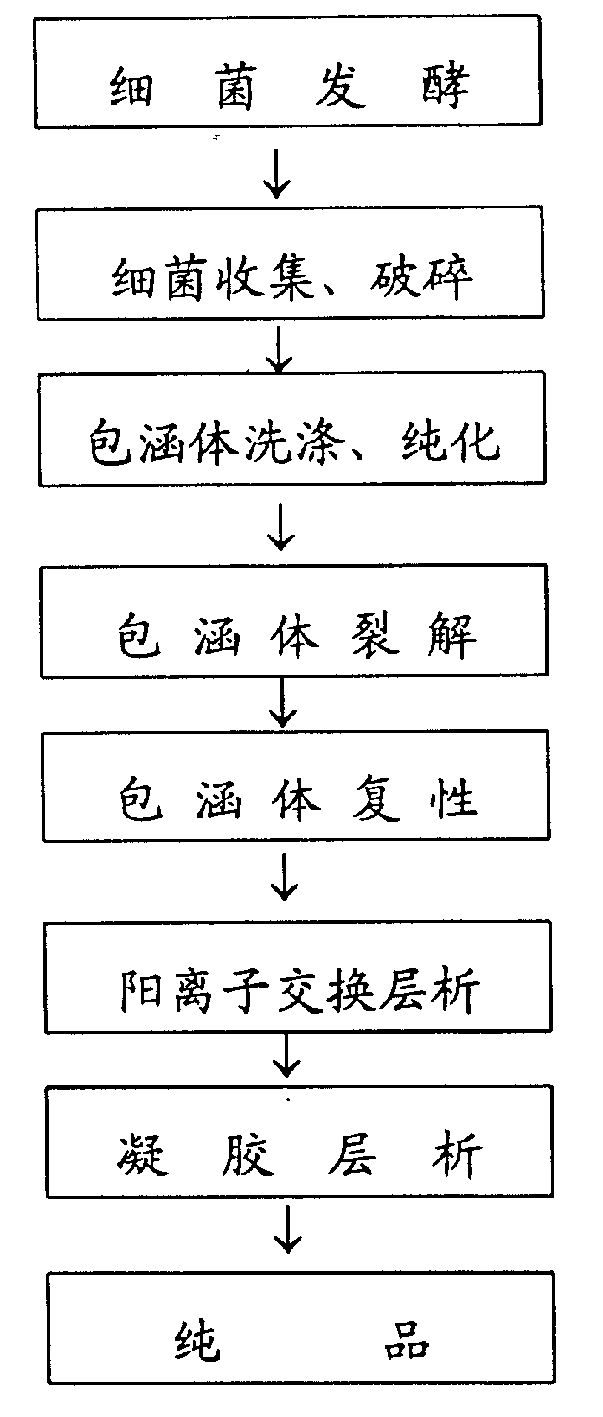
[0033] 1. Bacteria are fermented at low density;
[0034] 2. The inclusion body denaturant is urea;
[0035] 3. Purify the inclusion body by cation exchange chromatography after renaturation;
[0036] 2. Operation steps;
[0037] 1. Activation of engineering strains: Recombinant pBV220-hALRL / JM109 was constructed in our laboratory. Pick a well-separated single colony from the bacterial plate preserved at 4°C, inoculate it in 5ml LB / Amp (100ug / ml) medium, shake and cultivate at 200rpm at 30°C for 12h; Shake culture at 200 rpm at 30°C for 12 hours as seed solution.
[0038] 2. Fermentation: Inoculate the seed liquid in 500ml LB / Amp (100ug / ml) medium according to 2.5%, shake and culture at 200rpm at 30°C for 3-4h, wait for the OD 600 When =0.3-0.4, raise the culture temperature to 42°C, and continue shaking cultu...
Embodiment 2
[0045] Example 2: Recombinant full-length human enhancer of liver regeneration promotes DNA synthesis of the hepatic cell line HepG2 in vitro
[0046] One, the technical points of this embodiment:
[0047] Recombinant full-length human enhancer of liver regeneration promotes DNA synthesis in the hepatic cell line HepG2 in vitro.
[0048] 2. Operation steps
[0049] 1. HepG2 cell culture: HepG2 cells were grown in high-glucose DMEM+10% fetal bovine serum, 37°C, 5% CO 2 to cultivate. After the cells grow to the logarithmic phase, trypsinize and adjust the cell density to 4×10 5 / ml, inoculate 100ul per well in a 96-well culture plate, and continue culturing for 6h.
[0050] 2. Adding samples: Add the samples to be tested in the 96-well culture plate, set 3 replicate wells for each concentration, and continue to culture for 24 hours. If 2.0×10 4 Q 3 H-TdR, continue to culture for 3h.
[0051] 3. Detection: Use the cell harvester to collect the cells on the filter paper, a...
Embodiment 3
[0053] Example 3: Recombinant full-length human enhancer of liver regeneration promotes DNA synthesis in rat liver cells after partial hepatectomy in vivo
[0054] One, the technical points of this embodiment:
[0055] Recombinant full-length human enhancer of liver regeneration promotes DNA synthesis in rat liver cells after partial hepatectomy in vivo.
[0056] 2. Operation steps:
[0057] 1. Establishment of rat partial hepatectomy model: Rats were reared in a single cage for 1wk, fasted overnight before the experiment, and pentobarbital sodium (40mg kg -1 ) anesthetized with 750mL·L after epigastric hair removal -1 Disinfect the skin with alcohol, make a midline incision in the upper abdomen, and expose the liver. After the ligaments of the left and middle lobes of the liver are fully freed according to Hoggin’s method, the roots of each liver lobe are firmly ligated, and the left and middle lobes of the liver are removed quickly at the distal end of the ligature. That ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com