Composite zirconium-cerium oxide, process for the preparation thereof, and cocatalyst for cleaning exhaust gas
A technology for composite oxides and manufacturing methods, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, rare earth metal oxides/hydroxides, etc., can solve the problem of specific surface area reduction, mixing , Inappropriate and other problems, to achieve good reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
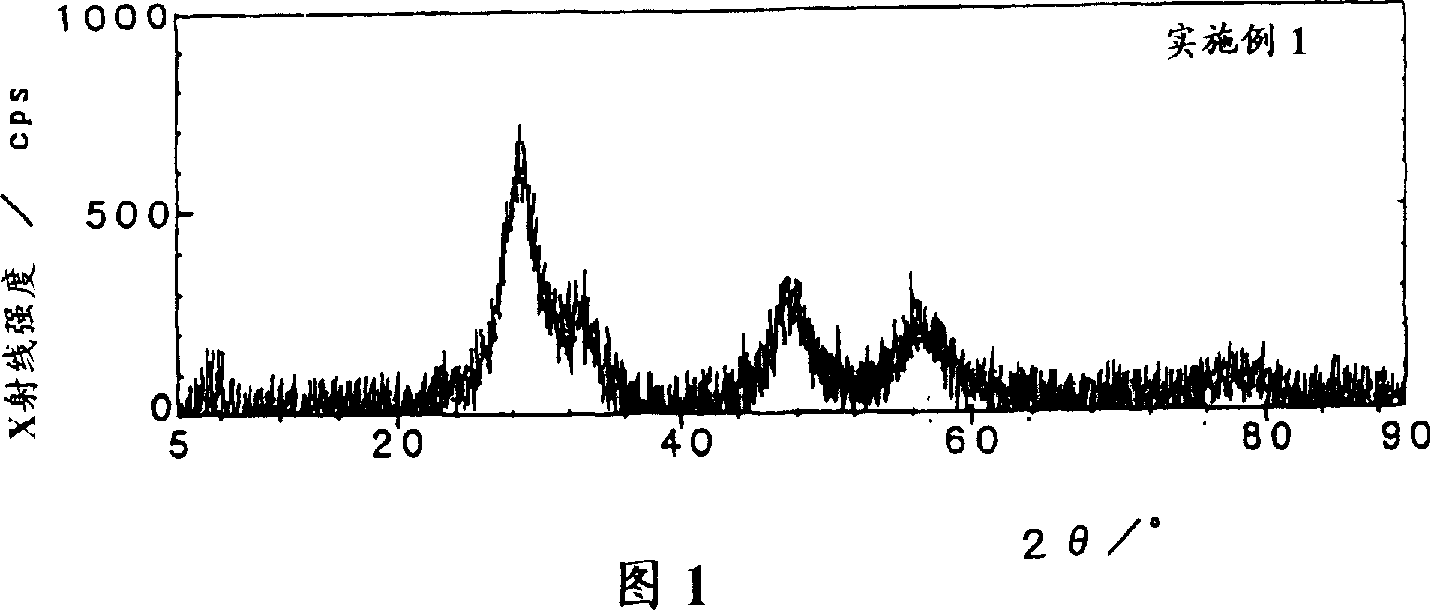
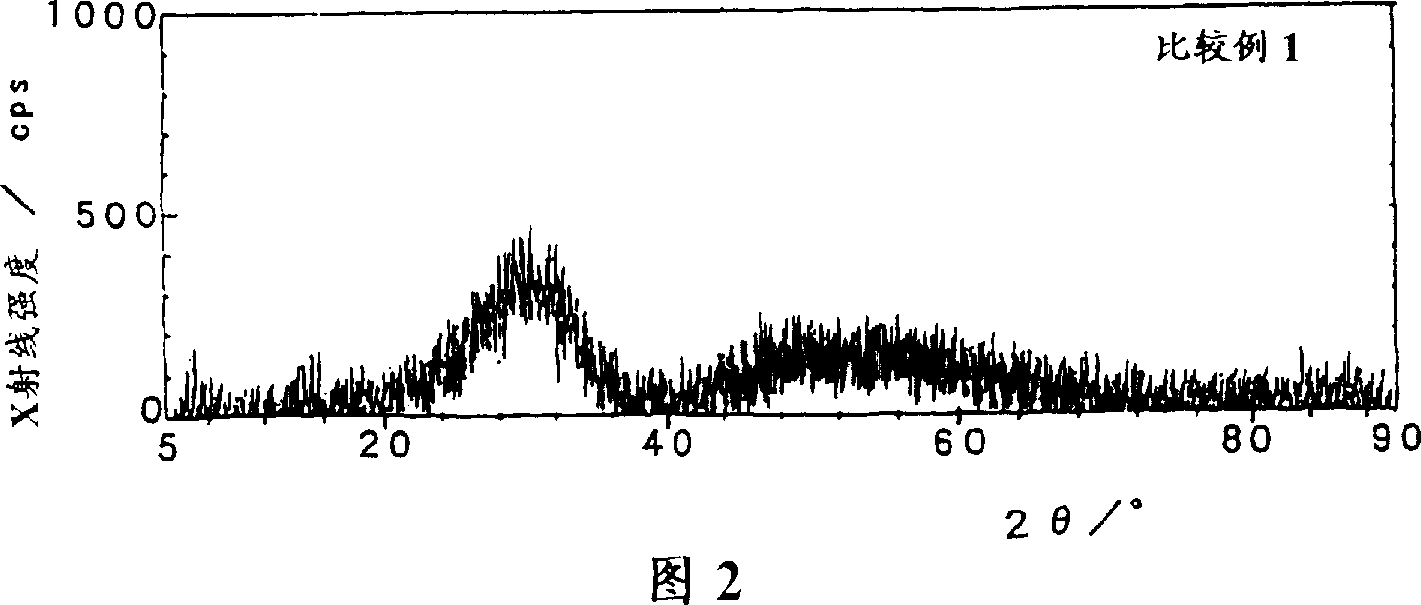
[0044] 81.05 g of zirconium hydroxide powder (manufactured by Santoku Metal Industry Co., Ltd., purity 99.9%, converted to zirconium hydroxide content of 40.1% by weight, average particle diameter of 18.56 μm), 150 ml of cerium sol (ロ-ヌ·プ- 16.67ml of lanthanum nitrate aqueous solution (manufactured by Anan Kasei Co., Ltd., purity 99.9%, 150g / liter converted to lanthanum oxide concentration). l) mixed to obtain ZrO 2 : CeO 2 : La 2 o 3 = 65:30:5 (weight ratio) mixture. Nitric acid (manufactured by Wako Pure Chemical Industries, Ltd., purity 60-61%) was added to the mixture so that its presence reached 6 times the total molar amount of cerium, and then the concentration in terms of oxides was adjusted to 50 g / liter with desalted water, 1 liter of mixture was obtained. Then 1 liter of this mixture was transferred to a vessel equipped with a steam cooling tube and heated at 100°C for 12 hours with stirring. After cooling slowly to 20°C, ammonia water (manufactured by Wako Pu...
Embodiment 2
[0046] 93.52g of zirconium hydroxide powder used in Example 1, 100ml of cerium sol and 16.67ml of lanthanum nitrate aqueous solution were mixed to obtain ZrO 2 : CeO 2 : La 2 o 3 =75:20:5 (weight ratio) mixture. Nitric acid was added to the mixture so that the amount was 6.2 times the molar amount of cerium, and the concentration in terms of oxide was adjusted to 50 g / liter with desalted water to obtain 1 liter of the mixture. Next, stirring and heating were carried out in the same manner as in Example 1, ammonia water was added, and the product was subjected to solid-liquid separation to obtain 142 g of precipitates. The obtained precipitate was subjected to X-ray diffraction measurement under the same conditions as in Example 1. The exact crystallite diameter could not be measured, and the X-ray intensity showing crystallinity was about 700 cps. Carry out firing similarly with embodiment 1, obtain specific surface area 115.7m 2 / g of zirconium-cerium-based lanthanum-co...
Embodiment 3
[0048] The zirconium hydroxide powder used in 93.52g embodiment 1 is mixed with 125ml cerium sol, obtains ZrO 2 : CeO 2 =75:25 (weight ratio) mixture. Nitric acid was added to the mixture in an amount of 5.5 times moles of the total molar amount of cerium, and the concentration was adjusted to 50 g / liter in terms of oxides with desalted water to obtain 1 liter of the mixture. Next, it operated similarly to Example 1, and obtained 141.62 g of precipitates. The obtained precipitate was subjected to X-ray diffraction measurement under the same conditions as in Example 1. The exact crystallite diameter could not be measured, and the X-ray intensity showing crystallinity was about 700 cps. Then operate in the same way as in Example 1 to obtain a specific surface area of 112.4m 2 / g of zirconium-cerium-based lanthanum-containing composite oxide 50g. Table 1 shows the specific surface areas when the obtained composite oxides were heated at 900°C, 1000°C, and 1100°C for 6 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com