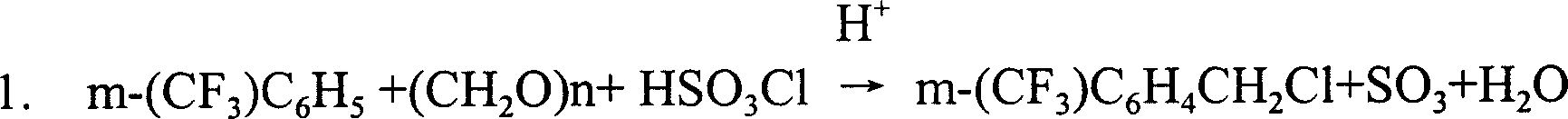Method for synthesizing m-trifluoromethyl benzyl cyanide
A technology of trifluoromethyl benzene acetonitrile and trifluoromethyl benzene is applied in the field of synthesis of organic fluorine compounds, and can solve the problems of environmental pollution, large chemical solvent, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 m-trifluoromethyl benzyl cyanide
[0022] In the reaction pot, add 100 kilograms of 85% sulfuric acid, 298 kilograms of 98% trifluoromethylbenzene (2.00kmol) and 90 kilograms of 98% paraformaldehyde (2.94kmol, melting point 64-67 ℃), at 15 ℃ ~ 25 ℃ Next, 265 kg of 98% chlorosulfonic acid (2.23 kmol) were added dropwise. After the dropwise addition, react at 40°C for 2 hours, remove the acidic aqueous phase after resting, wash twice with water, then neutralize to PH6 with 10% sodium hydroxide solution, carry out vacuum distillation at a vacuum degree of 0.092MPa, and collect 110 kilograms of trifluoromethylbenzene in front of 50~55 DEG C, content 96% (applicable), then collect 75~80 DEG C of fractions under vacuum degree of 0.099MPa, obtain 170 kilograms of m-trifluoromethyl chloromethylbenzenes, The content is 97.5%, and the yield is 66.73% (based on trifluoromethylbenzene). Residual liquid can make 3,3'-two (trifluoromethyl) phenyl metha...
Embodiment 2
[0034] The synthesis of embodiment 2 m-trifluoromethyl benzyl cyanide
[0035] In the reaction pot, add 100 kilograms of 75% sulfuric acid, 298 kilograms of 98% trifluoromethylbenzene (2.00kmol) and 90 kilograms of 98% paraformaldehyde (2.94kmol, melting point 121-123 ° C), at 15 ° C ~ 25 ° C Next, 265 kg of 98% chlorosulfonic acid (2.23 kmol) were added dropwise. After the dropwise addition, react at 55°C for 1 hour, remove the acidic aqueous phase after resting, wash twice with water, then neutralize to PH6 with 10% sodium hydroxide solution, carry out vacuum distillation at a vacuum of 0.092MPa, and collect 114 kg of trifluoromethylbenzene at 50-55°C, with a content of 96.5% (applicable), and 164 kg of m-trifluoromethylchloromethylbenzene at 75-80°C, with a content of 97.1 %, the yield is 65.7% (calculated in trifluoromethylbenzene). Residual liquid can make 3,3'-two (trifluoromethyl) phenyl methane 29 kilograms.
[0036] In the reaction pot, add 164 kilograms of 97.1% m...
Embodiment 3
[0038] Embodiment 3 The comparative example corresponding to the second step cyanation reaction of embodiment 1
[0039] In the reaction pot, add 170 kilograms of 97.5% m-trifluoromethyl chloromethylbenzene (0.85kmols), 165 kilograms of 33% sodium cyanide aqueous solution (1.11kmols) and 2 kilograms of tetrabutylammonium bromide to carry out cyanation reaction , reacted at 45°C for 12 hours, without mass tracking, and the impurity content of the organic phase was 5.5%. The water phase was separated, washed twice with water, and distilled under reduced pressure at a vacuum of 0.099MPa to collect fractions at 94-97°C. Obtain 146 kilograms of trifluoromethyl benzyl nitrile product, total content reaches 98.7%, and its middle-trifluoromethyl benzyl nitrile content reaches 94.0%, ortho-trifluoromethyl benzyl nitrile content is 1.4%, p-trifluoromethyl benzyl nitrile The content of phenylacetonitrile is 3.3%, and the reaction yield is 87.05% (calculated as m-trifluoromethylchlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com