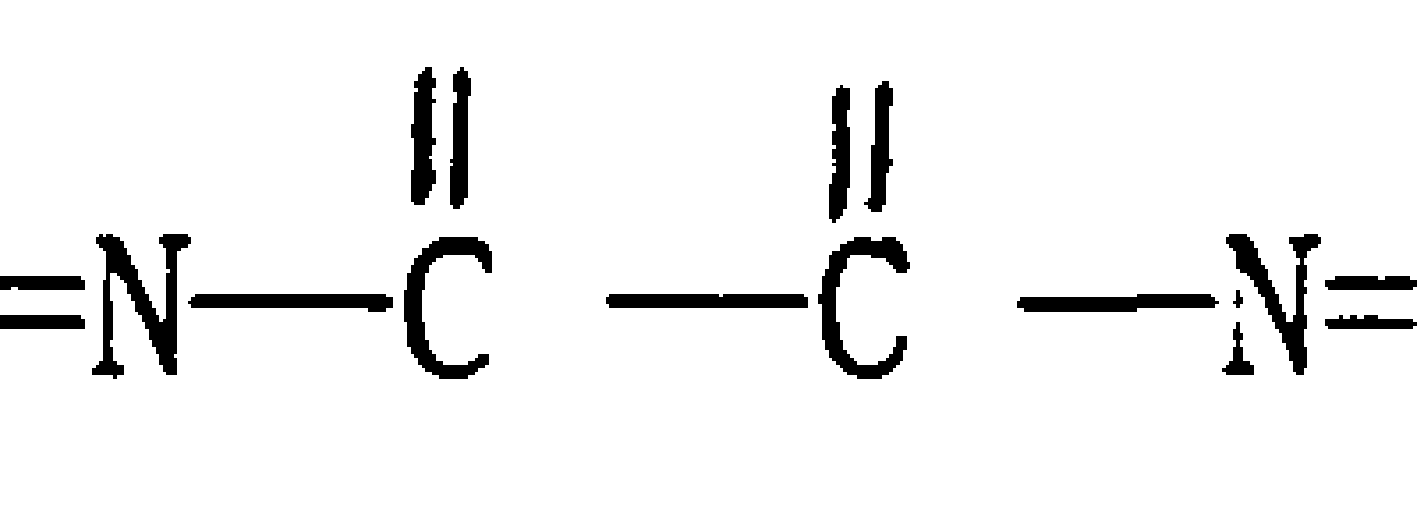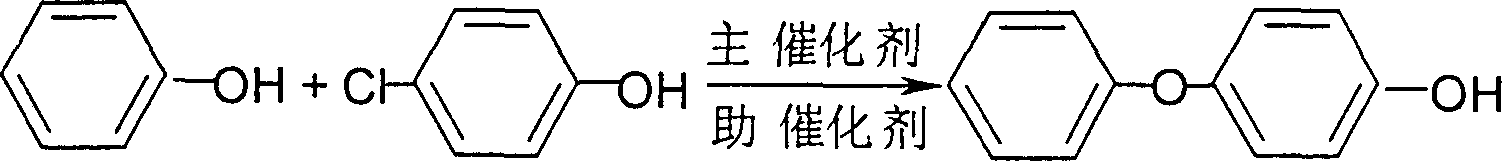New technology of synthesizing fenoxycarb
A technology of fenoxycarb and a new process, which is applied in the new process field of synthesizing fenoxycarb, can solve the problems of decreased catalytic activity and selectivity, restrictions on the popularization and use of fenoxycarb, and high reaction temperature, and can overcome the long reaction time and the high reaction temperature. The effect of shortening the time and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a four-neck flask equipped with stirring, a thermometer and a water separator, add 700 g of phenol, 84.0 g of potassium hydroxide, and 400 ml of toluene, heat and stir, and perform azeotropic dehydration. Add 6.0 g of the pre-prepared composite catalyst (weigh 4.0 g of 1,10-phenanthroline and dissolve in methanol, add 2.0 g of CuCl and stir for 1 hour until uniform, then evaporate the methanol). Heat up to 170°C and add 129 g of p-chlorophenol (1.0 mol) dropwise. After the addition is complete, react at 180°C for 3 hours. Cool to room temperature, adjust to pH = 2 with 10% HCl, separate the oil layer, wash with water until neutral, dry and distill under reduced pressure, first recover the solvent and phenol, collect the fraction at 145-148°C / 800Pa as the product 4-phenoxy Base phenol, a total of 131.0g, a purity of 94.5%, a yield of 70.4% (based on 1 mole of p-chlorophenol).
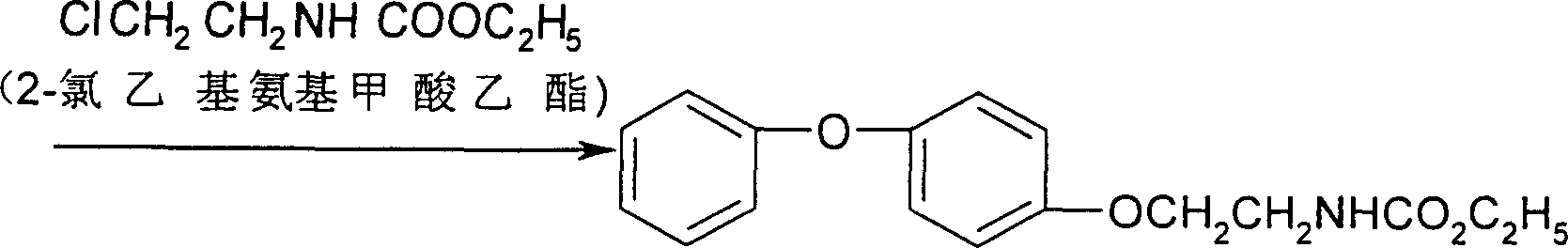
[0025] Use 4-phenoxyphenol to react with ethyl 2-chloroethyl carbamate to obtain fenoxyca...
Embodiment 2
[0027] Add 470g of phenol, 84.0g of potassium hydroxide, and 400ml of toluene to a four-necked flask equipped with stirring, a thermometer and a water separator, heat and stir for azeotropic dehydration. Add 4.0 g of the pre-prepared composite catalyst (weigh 2.5 g of 1,10-phenanthroline and dissolve it in methanol, add 1.5 g of CuCl and stir for 1 hour until uniform, then evaporate the methanol). Heat up to 170°C, add 129g of p-chlorophenol (1.0mol) dropwise, and continue reaction at 170°C for 4 hours after addition. The remaining operations were the same as in Example 1 to obtain 121.6 g of the product 4-phenoxyphenol with a purity of 95.5% and a yield of 65.4%.
[0028] Fenoxycarb is obtained by reacting 4-phenoxyphenol with ethyl 2-chloroethyl carbamate, the purity is ≥98%, and the yield is 95%.
Embodiment 3
[0030] Add 470g of phenol, 84.0g of potassium hydroxide, and 400ml of toluene into a four-neck flask equipped with stirring, a thermometer and a water separator, heat and stir, and perform azeotropic dehydration. Add the pre-prepared composite catalyst (weigh 4.5g 1,10-phenanthroline and 1.5g 2,2'-biquinoline and dissolve in methanol, add 3.5g CuBr and stir for 1 hour until uniform, distill off methanol). Heat up to 170°C, add 129g of p-chlorophenol (1.0mol) dropwise, and react at 180°C for 3 hours. The remaining operations were the same as in Example 1 to obtain 125.2 g of the product 4-phenoxyphenol with a purity of 95.7% and a yield of 67.5%.
[0031] Fenoxycarb is obtained by reacting 4-phenoxyphenol with ethyl 2-chloroethyl carbamate, the purity is ≥98%, and the yield is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com