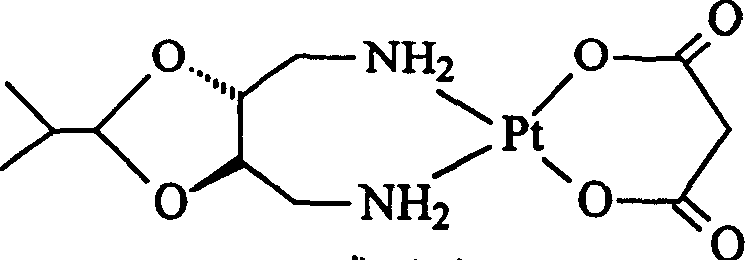
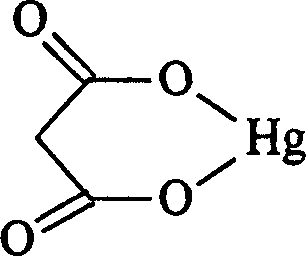
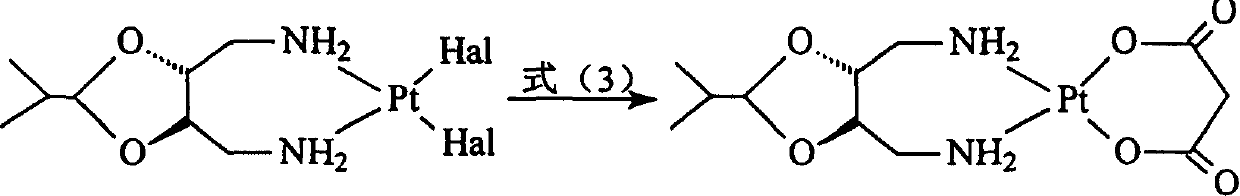
Method for preparing platinum(II) metal complex eptaplatin
A technology of metal complexes and complexes, which is applied to the active ingredients of heavy metal compounds, pharmaceutical formulations, chemical instruments and methods, etc., can solve the problems of difficult large-scale production, high product cost, expensive silver salts, etc., and achieves complete reaction, The effect of stable product quality and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Malonic acid (5.2 grams, 0.05 moles) was dissolved in 100 milliliters of water, and 30 milliliters of aqueous solutions of potassium hydroxide (5.6 grams, 0.1 moles) were added to adjust the pH of the solution to about 8, stirred at room temperature for 1 hour, and under nitrogen protection, Add Hg(NO 3 ) 2 1 / 2H 2 O (16.68 g, 0.05 mol) was dissolved in 100 ml of an aqueous solution, and the mixture was stirred at room temperature for 2 hours in the dark under the protection of nitrogen. Filter and dry under reduced pressure to obtain 13.6 g of mercuric malonate powder, with a yield of 89.9%, which is sealed and protected from light for future use.
[0047]cis-diiodo-[(4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane]platinum(II) (6.23 g, 10 mmol ) and mercuric malonate (3.03 g, 10 mmol) in 750 ml of aqueous solution were suspended and stirred for 20 hours at 55° C. in a dark room, and the resulting mercuric iodide precipitate was filtered out. Add 10 milliliter...
Embodiment 2
[0057] cis-diiodo-[(4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane]platinum(II) (6.23 g, 10 mmol ) and the mercurous malonate salt (3.03 grams, 10 mmoles) obtained in Example 1 were in 750 milliliters of aqueous solutions, suspended and stirred for 32 hours at room temperature in a dark room at 25° C., filtered out the precipitate of mercurous iodide generated, The filtrate was filtered with a microporous membrane (0.22 μm). The filtrate was concentrated under reduced pressure until a large amount of white crystals were precipitated, filtered, and dried to obtain 3.56 g of eplatin, with a yield of 75.5%. The crude product was determined by HPLC: 93.8%.
Embodiment 3
[0059] cis-diiodo-[(4R,5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane]platinum(II) (6.23 g, 10 mmol ) and the mercurous malonate salt (3.03 grams, 10 mmoles) obtained in Example 1 were in 750 milliliters of aqueous solutions, suspended and stirred for 12 hours at 80° C. in a dark room, filtered out the precipitate of mercurous iodide generated, and the filtrate Then filter with a microporous membrane filter (0.22 μm). The filtrate was concentrated under reduced pressure until a large amount of white crystals precipitated, filtered, and dried to obtain 3.92 g of Epplatin, with a yield of 83.2%. The crude product was determined by HPLC: 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com